| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| host | rabbit |
| isotype | IgG |
| clonality | monoclonal |
| concentration | 0.1 mg/mL |
| applications | Flow Cyt, IHC, IP, WB |
| reactivity | human, mouse |
| available sizes | 100 µL, 20 µL |
rabbit anti-p53 polyclonal antibody bl2501h11 1023
Price range: $100.00 through $300.00
Antibody summary
- Rabbit polyclonal to p53
- Suitable for: IHC, IP, WB, Flow Cyt
- Reacts with: Hu, Ms
- Isotype: IgG
- 100 µL (10 blots), 20 µL
rabbit anti- p53 polyclonal antibody bl2501h11 1023
| antibody |
|---|
| Database link: human P04637 mouse P02340 |
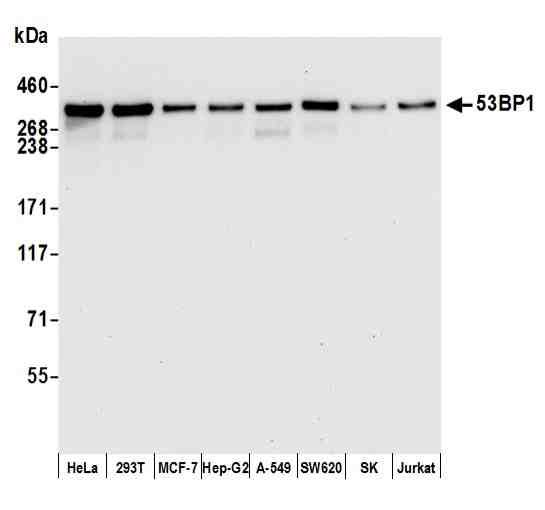
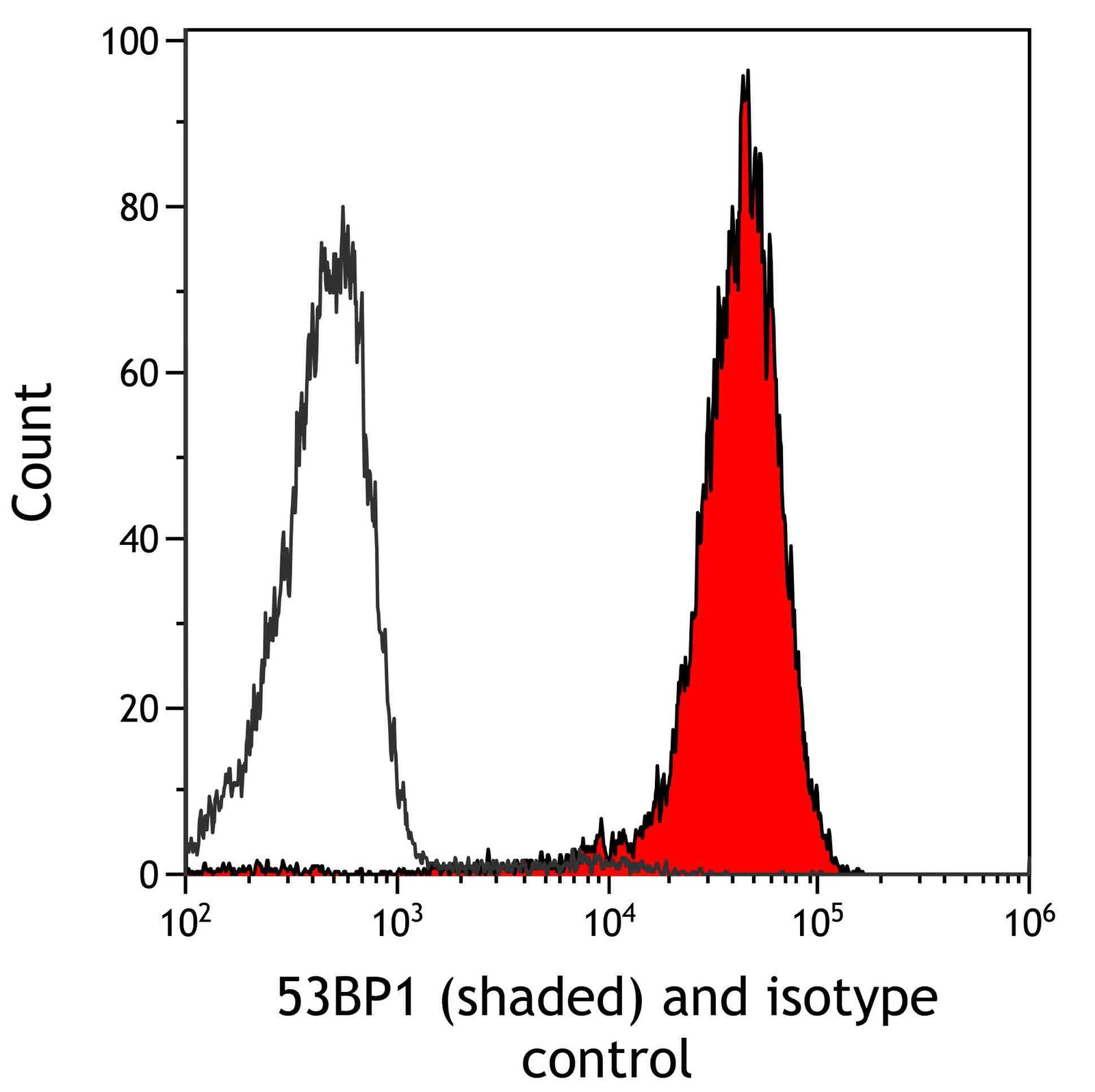
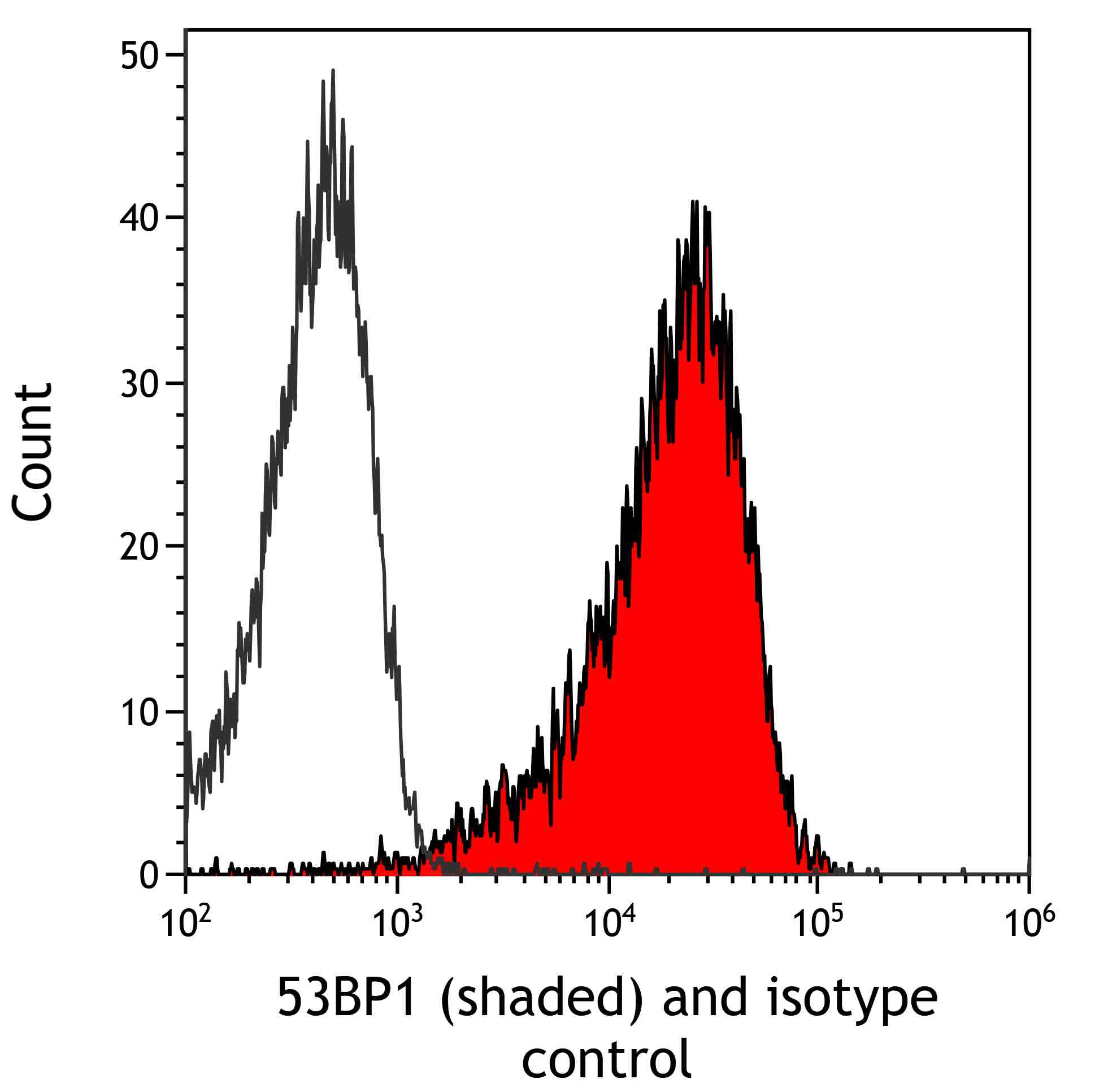
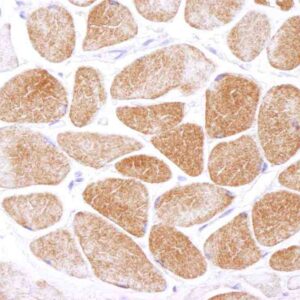
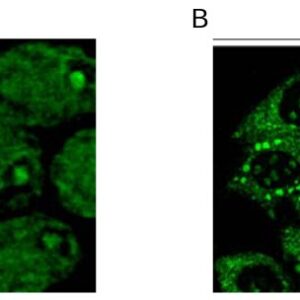
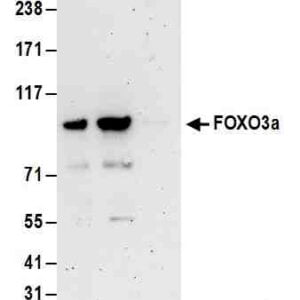
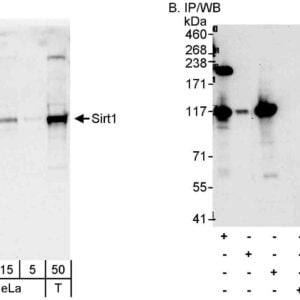
| Tested applications IHC, IP, WB, Flow Cyt |
| Recommended dilutions Flow Cytometry (Flow Cyt) Fixed in 4% formaldehyde and permeabilized with 90% methanol. 1 µl per 1 x 10^6 cells.,Immunohistochemistry (IHC) 1:100 to 1:500. Epitope retrieval with citrate buffer pH6.0 is recommended for FFPE tissue sections.,Immunoprecipitation (IP) 20 µl/mg lysate,Wester |
| Immunogen Between 350 and 400 |
| Size and concentration 100µL and 0.1 mg/mL |
| Form liquid |
| Storage Instructions Store at 2-8°C. Expires 1 year from date of receipt. |
| Storage buffer Tris-buffered Saline containing 0.1% BSA and 0.09% Sodium Azide |
| Purity affinity purified |
| Clonality monoclonal |
| Isotype IgG |
| Compatible secondaries goat anti-rabbit IgG, H&L chain specific, peroxidase conjugated, conjugated polyclonal antibody 9512 goat anti-rabbit IgG, H&L chain specific, biotin conjugated polyclonal antibody 2079 goat anti-rabbit IgG, H&L chain specific, FITC conjugated polyclonal antibody 7863 goat anti-rabbit IgG, H&L chain specific, Cross Absorbed polyclonal antibody 2371 goat anti-rabbit IgG, H&L chain specific, biotin conjugated polyclonal antibody, crossabsorbed 1715 goat anti-rabbit IgG, H&L chain specific, FITC conjugated polyclonal antibody, crossabsorbed 1720 |
| Isotype control Rabbit monoclonal - Isotype Control |
| target relevance |
|---|
| Protein names Cellular tumor antigen p53 (Antigen NY-CO-13) (Phosphoprotein p53) (Tumor suppressor p53) |
| Gene names TP53,TP53 P53 |
| Mass 43653Da |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| Western blot IHC ICC |
Documents
| # | SDS | Certificate | |
|---|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | |||
Only logged in customers who have purchased this product may leave a review.



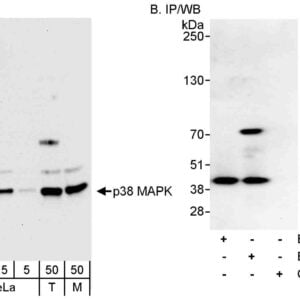
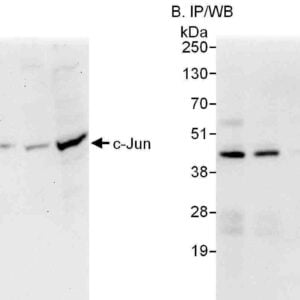
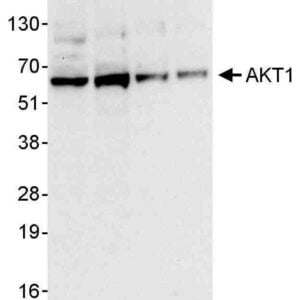
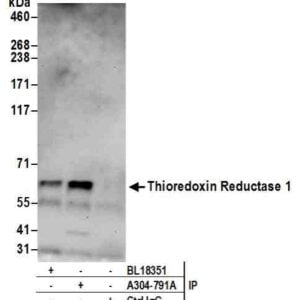

Reviews
There are no reviews yet.