| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| host | mouse |
| isotype | IgG |
| clonality | monoclonal |
| concentration | concentrate, predilute |
| applications | IHC |
| reactivity | human |
| available size | 0.1 mL, 0.5 mL, 1 mL concentrated, 7 mL prediluted |
rabbit anti-HBsAg monoclonal antibody (ZR393) 6209
Price range: $160.00 through $528.00
Antibody summary
- Rabbit monoclonal to Hepatitis B Virus (HBV) HBsAg
- Suitable for: Immunohistochemistry (formalin-fixed, paraffin-embedded tissues)
- Reacts with: Human
- Isotype:IgG
- Control: Hepatitis B infected liver
- Visualization: Cytoplasmic
- 0.1, 0.5, 1.0 mL concentrated, 7 mL prediluted
rabbit anti-HBsAg monoclonal antibody ZR393 6209
| target relevance |
|---|
| Protein names Large envelope protein (L glycoprotein) (L-HBsAg) (LHB) (Large S protein) (Large surface protein) (Major surface antigen) |
| Gene names S,S |
| Protein family Orthohepadnavirus major surface antigen family |
| Mass 43669Da |
| Function FUNCTION: The large envelope protein exists in two topological conformations, one which is termed 'external' or Le-HBsAg and the other 'internal' or Li-HBsAg. In its external conformation the protein attaches the virus to cell receptors and thereby initiating infection. This interaction determines the species specificity and liver tropism. This attachment induces virion internalization predominantly through caveolin-mediated endocytosis. The large envelope protein also assures fusion between virion membrane and endosomal membrane. In its internal conformation the protein plays a role in virion morphogenesis and mediates the contact with the nucleocapsid like a matrix protein. {ECO:0000255|HAMAP-Rule:MF_04075}.; FUNCTION: The middle envelope protein plays an important role in the budding of the virion. It is involved in the induction of budding in a nucleocapsid independent way. In this process the majority of envelope proteins bud to form subviral lipoprotein particles of 22 nm of diameter that do not contain a nucleocapsid. {ECO:0000255|HAMAP-Rule:MF_04075}. |
| Subellular location SUBCELLULAR LOCATION: Virion membrane {ECO:0000255|HAMAP-Rule:MF_04075}. |
| Structure SUBUNIT: [Isoform L]: In its internal form (Li-HBsAg), interacts with the capsid protein and with the isoform S. Interacts with host chaperone CANX. {ECO:0000250|UniProtKB:P03141}.; SUBUNIT: [Isoform M]: Associates with host chaperone CANX through its pre-S2 N glycan; this association may be essential for isoform M proper secretion. {ECO:0000250|UniProtKB:P03141}.; SUBUNIT: [Isoform S]: Interacts with isoform L. Interacts with the antigens of satellite virus HDV (HDVAgs); this interaction is required for encapsidation of HDV genomic RNA. {ECO:0000250|UniProtKB:P03141}. |
| Post-translational modification PTM: Isoform M is N-terminally acetylated by host at a ratio of 90%, and N-glycosylated by host at the pre-S2 region. {ECO:0000250|UniProtKB:P03138, ECO:0000255|HAMAP-Rule:MF_04075}.; PTM: Myristoylated. {ECO:0000255|HAMAP-Rule:MF_04075}. |
| Domain DOMAIN: The large envelope protein is synthesized with the pre-S region at the cytosolic side of the endoplasmic reticulum and, hence will be within the virion after budding. Therefore the pre-S region is not N-glycosylated. Later a post-translational translocation of N-terminal pre-S and TM1 domains occur in about 50% of proteins at the virion surface. These molecules change their topology by an unknown mechanism, resulting in exposure of pre-S region at virion surface. For isoform M in contrast, the pre-S2 region is translocated cotranslationally to the endoplasmic reticulum lumen and is N-glycosylated. {ECO:0000255|HAMAP-Rule:MF_04075}. |
| Biotechnology BIOTECHNOLOGY: Systematic vaccination of individuals at risk of exposure to the virus has been the main method of controlling the morbidity and mortality associated with hepatitis B. The first hepatitis B vaccine was manufactured by the purification and inactivation of HBsAg obtained from the plasma of chronic hepatitis B virus carriers. The vaccine is now produced by recombinant DNA techniques and expression of the S isoform in yeast cells. The pre-S region do not seem to induce strong enough antigenic response. |
| Target Relevance information above includes information from UniProt accession: Q76R62 |
| The UniProt Consortium |
Data
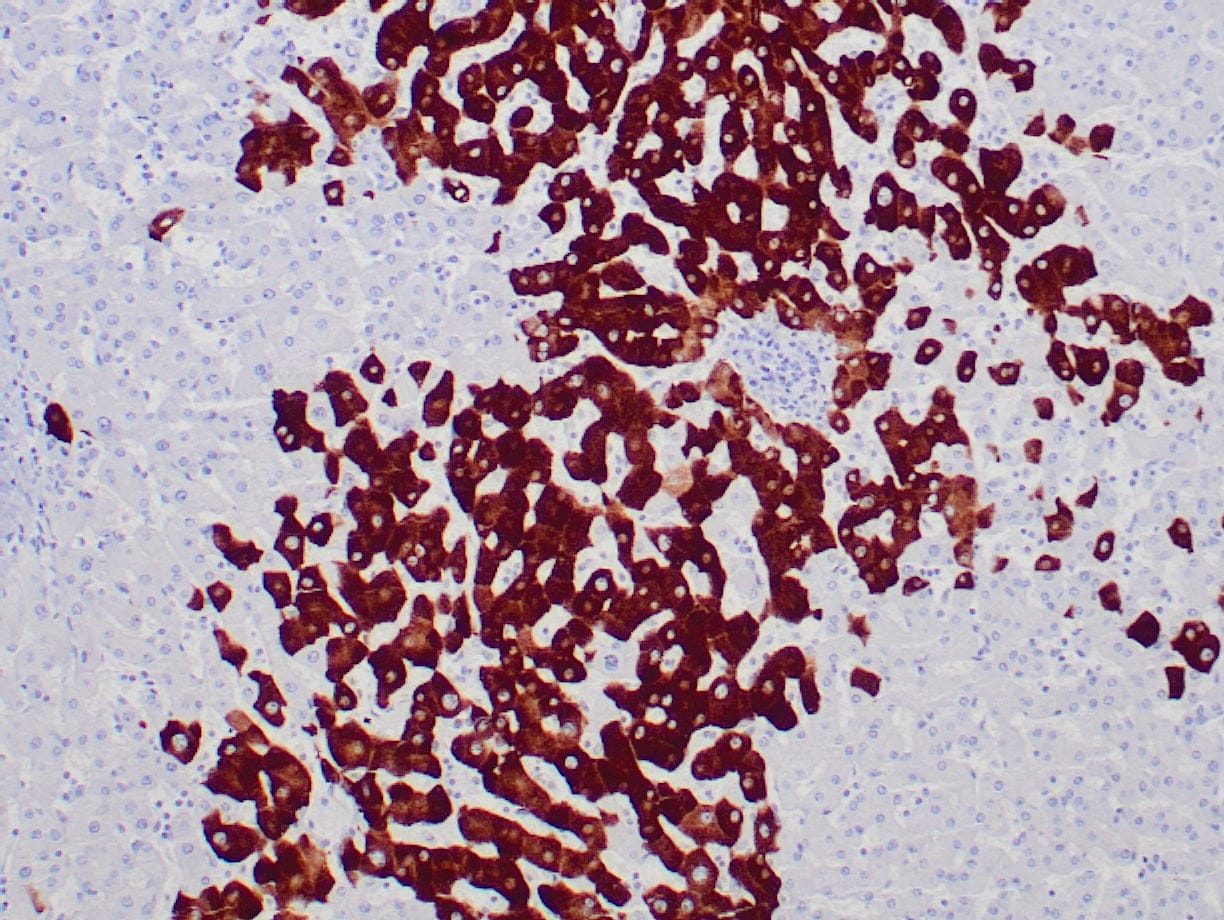 |
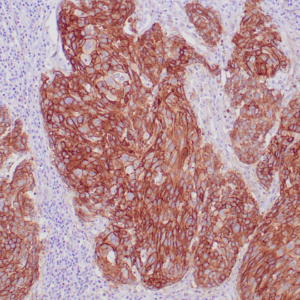
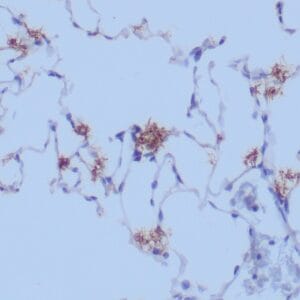
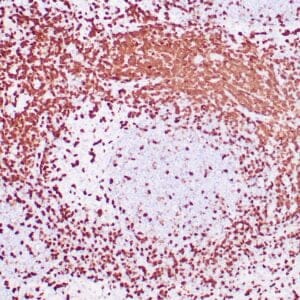
| Human liver infected by hepatitis B virus stained with anti-HBsAg antibody using peroxidase-conjugate and DAB chromogen. Note the cytoplasmic staining of infected hepatocytes. |
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| IHC |
Documents
| # | SDS | Certificate | |
|---|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | |||
Only logged in customers who have purchased this product may leave a review.






Reviews
There are no reviews yet.