| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| host | rabbit |
| isotype | IgG |
| clonality | polyclonal |
| concentration | 50 µg/mL |
| applications | IHC |
| reactivity | human, mouse |
| available sizes | 10 µL, 100 µL |
rabbit anti-AMPK1 polyclonal antibody 1034
Price range: $121.00 through $300.00
Antibody summary
- Rabbit polyclonal to AMPK (AMP-activated protein kinase)
- Suitable for: IHC
- Reacts with: Hu, Ms presumed Guinea Pig, Horse, Zebra Finch, Turkey, Dog, Chicken, Pig, Rabbit, Bovine, Monkey, Rat
- Isotype: IgG
- 100 µL (50 slides), 10 µL (5 slides)
rabbit anti- ampk polyclonal antibody 1034
| target relevance |
|---|
| Protein names 5'-AMP-activated protein kinase catalytic subunit alpha-1 (AMPK subunit alpha-1) (EC 2.7.11.1) (Acetyl-CoA carboxylase kinase) (ACACA kinase) (Hydroxymethylglutaryl-CoA reductase kinase) (HMGCR kinase) (EC 2.7.11.31) (Tau-protein kinase PRKAA1) (EC 2.7.11.26) |
| Gene names PRKAA1,PRKAA1 AMPK1 |
| Protein family Protein kinase superfamily, CAMK Ser/Thr protein kinase family, SNF1 subfamily |
| Mass 64009Da |
| Function FUNCTION: Catalytic subunit of AMP-activated protein kinase (AMPK), an energy sensor protein kinase that plays a key role in regulating cellular energy metabolism (PubMed:17307971, PubMed:17712357, PubMed:24563466, PubMed:37821951). In response to reduction of intracellular ATP levels, AMPK activates energy-producing pathways and inhibits energy-consuming processes: inhibits protein, carbohydrate and lipid biosynthesis, as well as cell growth and proliferation (PubMed:17307971, PubMed:17712357). AMPK acts via direct phosphorylation of metabolic enzymes, and by longer-term effects via phosphorylation of transcription regulators (PubMed:17307971, PubMed:17712357). Regulates lipid synthesis by phosphorylating and inactivating lipid metabolic enzymes such as ACACA, ACACB, GYS1, HMGCR and LIPE; regulates fatty acid and cholesterol synthesis by phosphorylating acetyl-CoA carboxylase (ACACA and ACACB) and hormone-sensitive lipase (LIPE) enzymes, respectively (By similarity). Promotes lipolysis of lipid droplets by mediating phosphorylation of isoform 1 of CHKA (CHKalpha2) (PubMed:34077757). Regulates insulin-signaling and glycolysis by phosphorylating IRS1, PFKFB2 and PFKFB3 (By similarity). AMPK stimulates glucose uptake in muscle by increasing the translocation of the glucose transporter SLC2A4/GLUT4 to the plasma membrane, possibly by mediating phosphorylation of TBC1D4/AS160 (By similarity). Regulates transcription and chromatin structure by phosphorylating transcription regulators involved in energy metabolism such as CRTC2/TORC2, FOXO3, histone H2B, HDAC5, MEF2C, MLXIPL/ChREBP, EP300, HNF4A, p53/TP53, SREBF1, SREBF2 and PPARGC1A (PubMed:11518699, PubMed:11554766, PubMed:15866171, PubMed:17711846, PubMed:18184930). Acts as a key regulator of glucose homeostasis in liver by phosphorylating CRTC2/TORC2, leading to CRTC2/TORC2 sequestration in the cytoplasm (By similarity). In response to stress, phosphorylates 'Ser-36' of histone H2B (H2BS36ph), leading to promote transcription (By similarity). Acts as a key regulator of cell growth and proliferation by phosphorylating FNIP1, TSC2, RPTOR, WDR24 and ATG1/ULK1: in response to nutrient limitation, negatively regulates the mTORC1 complex by phosphorylating RPTOR component of the mTORC1 complex and by phosphorylating and activating TSC2 (PubMed:14651849, PubMed:18439900, PubMed:20160076, PubMed:21205641). Also phosphorylates and inhibits GATOR2 subunit WDR24 in response to nutrient limitation, leading to suppress glucose-mediated mTORC1 activation (PubMed:36732624). In response to energetic stress, phosphorylates FNIP1, inactivating the non-canonical mTORC1 signaling, thereby promoting nuclear translocation of TFEB and TFE3, and inducing transcription of lysosomal or autophagy genes (PubMed:37079666). In response to nutrient limitation, promotes autophagy by phosphorylating and activating ATG1/ULK1 (PubMed:21205641). In that process, it also activates WDR45/WIPI4 (PubMed:28561066). Phosphorylates CASP6, thereby preventing its autoprocessing and subsequent activation (PubMed:32029622). In response to nutrient limitation, phosphorylates transcription factor FOXO3 promoting FOXO3 mitochondrial import (By similarity). Also acts as a regulator of cellular polarity by remodeling the actin cytoskeleton; probably by indirectly activating myosin (PubMed:17486097). AMPK also acts as a regulator of circadian rhythm by mediating phosphorylation of CRY1, leading to destabilize it (By similarity). May regulate the Wnt signaling pathway by phosphorylating CTNNB1, leading to stabilize it (By similarity). Also has tau-protein kinase activity: in response to amyloid beta A4 protein (APP) exposure, activated by CAMKK2, leading to phosphorylation of MAPT/TAU; however the relevance of such data remains unclear in vivo (By similarity). Also phosphorylates CFTR, EEF2K, KLC1, NOS3 and SLC12A1 (PubMed:12519745, PubMed:20074060). Regulates hepatic lipogenesis. Activated via SIRT3, represses sterol regulatory element-binding protein (SREBP) transcriptional activities and ATP-consuming lipogenesis to restore cellular energy balance. Upon stress, regulates mitochondrial fragmentation through phosphorylation of MTFR1L (PubMed:36367943). {ECO:0000250|UniProtKB:P54645, ECO:0000250|UniProtKB:Q5EG47, ECO:0000269|PubMed:11518699, ECO:0000269|PubMed:11554766, ECO:0000269|PubMed:12519745, ECO:0000269|PubMed:14651849, ECO:0000269|PubMed:15866171, ECO:0000269|PubMed:17486097, ECO:0000269|PubMed:17711846, ECO:0000269|PubMed:18184930, ECO:0000269|PubMed:18439900, ECO:0000269|PubMed:20074060, ECO:0000269|PubMed:20160076, ECO:0000269|PubMed:21205641, ECO:0000269|PubMed:24563466, ECO:0000269|PubMed:28561066, ECO:0000269|PubMed:32029622, ECO:0000269|PubMed:34077757, ECO:0000269|PubMed:36367943, ECO:0000269|PubMed:36732624, ECO:0000269|PubMed:37079666, ECO:0000269|PubMed:37821951, ECO:0000303|PubMed:17307971, ECO:0000303|PubMed:17712357}. |
| Catalytic activity CATALYTIC ACTIVITY: Reaction=L-seryl-[protein] + ATP = O-phospho-L-seryl-[protein] + ADP + H(+); Xref=Rhea:RHEA:17989, Rhea:RHEA-COMP:9863, Rhea:RHEA-COMP:11604, ChEBI:CHEBI:15378, ChEBI:CHEBI:29999, ChEBI:CHEBI:30616, ChEBI:CHEBI:83421, ChEBI:CHEBI:456216; EC=2.7.11.1; Evidence={ECO:0000269|PubMed:32029622, ECO:0000269|PubMed:36732624, ECO:0000269|PubMed:37079666}; CATALYTIC ACTIVITY: Reaction=L-threonyl-[protein] + ATP = O-phospho-L-threonyl-[protein] + ADP + H(+); Xref=Rhea:RHEA:46608, Rhea:RHEA-COMP:11060, Rhea:RHEA-COMP:11605, ChEBI:CHEBI:15378, ChEBI:CHEBI:30013, ChEBI:CHEBI:30616, ChEBI:CHEBI:61977, ChEBI:CHEBI:456216; EC=2.7.11.1; Evidence={ECO:0000269|PubMed:24563466}; CATALYTIC ACTIVITY: Reaction=L-seryl-[acetyl-CoA carboxylase] + ATP = O-phospho-L-seryl-[acetyl-CoA carboxylase] + ADP + H(+); Xref=Rhea:RHEA:20333, Rhea:RHEA-COMP:13722, Rhea:RHEA-COMP:13723, ChEBI:CHEBI:15378, ChEBI:CHEBI:29999, ChEBI:CHEBI:30616, ChEBI:CHEBI:83421, ChEBI:CHEBI:456216; Evidence={ECO:0000250|UniProtKB:P54645}; CATALYTIC ACTIVITY: Reaction=L-seryl-[3-hydroxy-3-methylglutaryl-coenzyme A reductase] + ATP = O-phospho-L-seryl-[3-hydroxy-3-methylglutaryl-coenzyme A reductase] + ADP + H(+); Xref=Rhea:RHEA:23172, Rhea:RHEA-COMP:13692, Rhea:RHEA-COMP:13693, ChEBI:CHEBI:15378, ChEBI:CHEBI:29999, ChEBI:CHEBI:30616, ChEBI:CHEBI:83421, ChEBI:CHEBI:456216; EC=2.7.11.31; Evidence={ECO:0000250|UniProtKB:P54645}; CATALYTIC ACTIVITY: Reaction=L-seryl-[tau protein] + ATP = O-phospho-L-seryl-[tau protein] + ADP + H(+); Xref=Rhea:RHEA:12801, Rhea:RHEA-COMP:13701, Rhea:RHEA-COMP:13702, ChEBI:CHEBI:15378, ChEBI:CHEBI:29999, ChEBI:CHEBI:30616, ChEBI:CHEBI:83421, ChEBI:CHEBI:456216; EC=2.7.11.26; Evidence={ECO:0000250|UniProtKB:P54645}; CATALYTIC ACTIVITY: Reaction=L-threonyl-[tau protein] + ATP = O-phospho-L-threonyl-[tau protein] + ADP + H(+); Xref=Rhea:RHEA:53904, Rhea:RHEA-COMP:13703, Rhea:RHEA-COMP:13704, ChEBI:CHEBI:15378, ChEBI:CHEBI:30013, ChEBI:CHEBI:30616, ChEBI:CHEBI:61977, ChEBI:CHEBI:456216; EC=2.7.11.26; Evidence={ECO:0000250|UniProtKB:P54645}; |
| Subellular location SUBCELLULAR LOCATION: Cytoplasm {ECO:0000269|PubMed:15866171, ECO:0000269|PubMed:37821951}. Nucleus {ECO:0000269|PubMed:15866171}. Note=In response to stress, recruited by p53/TP53 to specific promoters. {ECO:0000269|PubMed:15866171}. |
| Structure SUBUNIT: AMPK is a heterotrimer of an alpha catalytic subunit (PRKAA1 or PRKAA2), a beta (PRKAB1 or PRKAB2) and a gamma non-catalytic subunits (PRKAG1, PRKAG2 or PRKAG3) (PubMed:21680840). Interacts with FNIP1 and FNIP2 (PubMed:17028174, PubMed:18403135, PubMed:18663353). {ECO:0000269|PubMed:17028174, ECO:0000269|PubMed:18403135, ECO:0000269|PubMed:18663353, ECO:0000269|PubMed:21680840}.; SUBUNIT: (Microbial infection) Interacts with Dengue type 2 virus non-structural protein 1; this interaction promotes the AMPK/ERK/mTOR signaling pathway to induce autophagy. {ECO:0000269|PubMed:37821951}. |
| Post-translational modification PTM: Ubiquitinated. {ECO:0000250|UniProtKB:Q5EG47}.; PTM: Phosphorylated at Thr-183 by STK11/LKB1 in complex with STE20-related adapter-alpha (STRADA) pseudo kinase and CAB39. Also phosphorylated at Thr-183 by CAMKK2; triggered by a rise in intracellular calcium ions, without detectable changes in the AMP/ATP ratio. CAMKK1 can also phosphorylate Thr-183, but at a much lower level. Dephosphorylated by protein phosphatase 2A and 2C (PP2A and PP2C). Phosphorylated by ULK1 and ULK2; leading to negatively regulate AMPK activity and suggesting the existence of a regulatory feedback loop between ULK1, ULK2 and AMPK. Dephosphorylated by PPM1A and PPM1B. {ECO:0000269|PubMed:14976552, ECO:0000269|PubMed:15980064, ECO:0000269|PubMed:16054095, ECO:0000269|PubMed:21460634}.; PTM: Glycosylated; O-GlcNAcylated by OGT, promoting the AMP-activated protein kinase (AMPK) activity. {ECO:0000269|PubMed:24563466}. |
| Domain DOMAIN: The AIS (autoinhibitory sequence) region shows some sequence similarity with the ubiquitin-associated domains and represses kinase activity. {ECO:0000269|PubMed:17088252, ECO:0000269|PubMed:9857077}. |
| Target Relevance information above includes information from UniProt accession: Q13131 |
| The UniProt Consortium |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| IHC |
Documents
| # | SDS | Certificate | |
|---|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | |||
Only logged in customers who have purchased this product may leave a review.


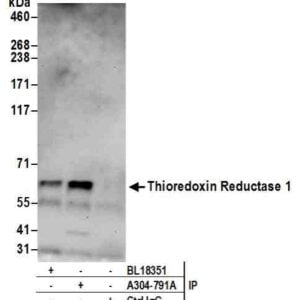
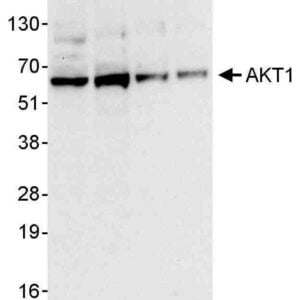
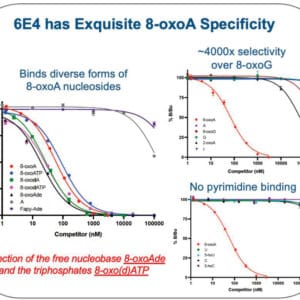

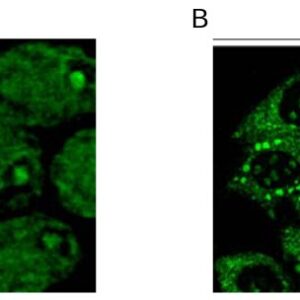
Reviews
There are no reviews yet.