| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| host | mouse |
| isotype | IgG1 |
| clonality | monoclonal |
| concentration | 1 mg/mL |
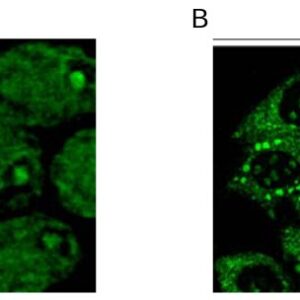
| applications | ICC/IF, WB |
| reactivity | Clostridium botulinum Toxin D |
| available sizes | 25 µg |
mouse anti-Clostridium botulinum Toxin D monoclonal antibody (5131) 7053
$469.00
Antibody summary
- Mouse monoclonal to Clostridium botulinum Toxin D
- Suitable for: ICC/IF,ELISA
- Isotype: IgG1
- 25 µg
mouse anti-Clostridium botulinum Toxin D monoclonal antibody (5131) 7053
| antibody |
|---|
| Tested applications ICC/IF,ELISA |
| Recommended dilutions ELISA: use a dilution of 1:20-1:200. Immunocytochemistry/ Immunohistochemistry: use a dilution of 1:10-1:50. Immunofluorescence: use a dilution of 1:10-1:50 Immunoblotting: use a dilution of 1:100-1:1,000. A band of ~150,000 is detected. End users should determine op |
| Immunogen Toxin D purified from culture filtrate of C. botulinum. |
| Size and concentration 25µg and lot specific |
| Form liquid |
| Storage Instructions This antibody is stable at 4°C for short-term storage and for at least one (1) year at -20° to -70°C. Store product in appropriate aliquots to avoid multiple freeze-thaw cycles. |
| Storage buffer PBS, pH 7.2, 0.1% NaN3. |
| Purity immunogen affinity purification |
| Clonality monoclonal |
| Isotype IgG1 |
| Compatible secondaries goat anti-mouse IgG, H&L chain specific, peroxidase conjugated polyclonal antibody 5486 goat anti-mouse IgG, H&L chain specific, biotin conjugated, Conjugate polyclonal antibody 2685 goat anti-mouse IgG, H&L chain specific, FITC conjugated polyclonal antibody 7854 goat anti-mouse IgG, H&L chain specific, peroxidase conjugated polyclonal antibody, crossabsorbed 1706 goat anti-mouse IgG, H&L chain specific, biotin conjugated polyclonal antibody, crossabsorbed 1716 goat anti-mouse IgG, H&L chain specific, FITC conjugated polyclonal antibody, crossabsorbed 1721 |
| Isotype control Mouse monocolonal IgG1 - Isotype Control |
| target relevance |
|---|
| Protein names Botulinum neurotoxin type D (BoNT/D) (Bontoxilysin-D) [Cleaved into: Botulinum neurotoxin D light chain (LC) (EC 3.4.24.69); Botulinum neurotoxin D heavy chain (HC)] |
| Gene names botD,botD |
| Protein family Peptidase M27 family |
| Mass 146872Da |
| Function FUNCTION: [Botulinum neurotoxin type D]: Botulinum toxin causes flaccid paralysis by inhibiting neurotransmitter (acetylcholine) release from the presynaptic membranes of nerve terminals of the eukaryotic host skeletal and autonomic nervous system, with frequent heart or respiratory failure (PubMed:16252491, PubMed:8175689). Precursor of botulinum neurotoxin D for which a proteinaceous coreceptor is controversial. In double SV2A/SV2B knockout mice this toxin does not degrade its synaptobrevin target; introducing SV2A, SV2B or SV2C restores target cleavage (PubMed:21483489). Recognition of SV2 by this toxin does not occur via SV2 glycosylation or its large extracellular loop 4 (PubMed:21483489). Another group does not find a convincing interaction with SV2 (PubMed:21632541). Thus a protein receptor for this BoNT serotype has yet to be definitively proven. Recognizes at least 1 complex polysialylated ganglioside found on neural tissue. Electrical stimulation increases uptake of toxin in an ex vivo assay, presumably by transiently exposing a receptor usually found in eukaryotic target synaptic vesicles (PubMed:19650874, PubMed:21483489, PubMed:21632541). Upon synaptic vesicle recycling the toxin is taken up via the endocytic pathway; when the pH of the toxin-containing endosome drops a structural rearrangement occurs so that the N-terminus of the heavy chain (HC) forms pores that allows the light chain (LC) to translocate into the cytosol (By similarity). Once in the cytosol the disulfide bond linking the 2 subunits is reduced and LC cleaves its target protein on synaptic vesicles, preventing their fusion with the cytoplasmic membrane and thus neurotransmitter release (By similarity). Requires complex eukaryotic host polysialogangliosides for full neurotoxicity and for binding to neurons (PubMed:20704566, PubMed:21483489). {ECO:0000250|UniProtKB:P0DPI0, ECO:0000269|PubMed:16252491, ECO:0000269|PubMed:19650874, ECO:0000269|PubMed:20704566, ECO:0000269|PubMed:21483489, ECO:0000269|PubMed:21632541, ECO:0000269|PubMed:8175689, ECO:0000305}.; FUNCTION: [Botulinum neurotoxin D light chain]: Has proteolytic activity (PubMed:8175689, PubMed:8197120). After translocation into the eukaryotic host cytosol, inhibits neurotransmitter release by acting as a zinc endopeptidase that cleaves the '61-Lys-|-Leu-62' bond of synaptobrevin-1 (VAMP1), and the equivalent 'Lys-|-Leu' sites in VAMP2 and VAMP3 (PubMed:8175689). Cleaves the '49-Lys-|-Ile-50' bond of A.californica synaptobrevin (AC P35589) (PubMed:8197120). This chain probably has to be partially unfolded to translocate into the eukaryotic host cell cytosol (PubMed:15584922). {ECO:0000269|PubMed:8175689, ECO:0000269|PubMed:8197120, ECO:0000305|PubMed:15584922}.; FUNCTION: [Botulinum neurotoxin D heavy chain]: Responsible for host epithelial cell transcytosis, host nerve cell targeting and translocation of light chain (LC) into eukaryotic host cell cytosol. Composed of 3 subdomains; the translocation domain (TD), and N-terminus and C-terminus of the receptor-binding domain (RBD). The RBD is responsible for the adherence of the toxin to the eukaryotic target cell surface. The N-terminus of the TD wraps an extended belt around the perimeter of the LC, protecting Zn(2+) in the active site; it may also prevent premature LC dissociation from the translocation channel and protect toxin prior to translocation (PubMed:17907800). The TD inserts into synaptic vesicle membrane to allow translocation into the host cytosol (By similarity). The RBD binds eukaryotic host phosphatidylethanolamine, which may serve as toxin receptor (PubMed:16115873). Treatment of synaptosomes with proteinase K does not reduce HC binding, suggesting there is no protein receptor or it is protected from extracellular proteases (PubMed:16115873). HC significantly decreases uptake and toxicity of whole BoNT/D (PubMed:19650874, PubMed:21483489). HC also interferes with uptake of tetanus toxin (PubMed:19650874). Has 2 closely located carbohydrate-binding receptor sites and binds at least 1 GT1b ganglioside (PubMed:20704566). Bind gangliosides in the order GD2 > GT1b > GD1b (PubMed:21632541). Interacts with eukaryotic target protein SV2B (synaptic vesicle glycoprotein 2B) (PubMed:21483489). Expression of SV2A, SV2B or SV2C in mice knocked-out for the SV2 proteins restores entry of BoNT/D and cleavage of VAMP2, suggesting SV2 acts as its receptor (PubMed:21483489). Unlike BoNT/A and BoNT/E, toxin uptake is not mediated by large extracellular loop 4 of SV2 (PubMed:21483489). Another group finds very poor interaction with SV2 proteins, suggesting the possible protein receptor may not have been identified (PubMed:21632541). {ECO:0000250|UniProtKB:P0DPI0, ECO:0000269|PubMed:16115873, ECO:0000269|PubMed:19650874, ECO:0000269|PubMed:20704566, ECO:0000269|PubMed:21483489, ECO:0000269|PubMed:21632541, ECO:0000305|PubMed:17907800}. |
| Catalytic activity CATALYTIC ACTIVITY: Reaction=Limited hydrolysis of proteins of the neuroexocytosis apparatus, synaptobrevins, SNAP25 or syntaxin. No detected action on small molecule substrates.; EC=3.4.24.69; Evidence={ECO:0000269|PubMed:8175689, ECO:0000269|PubMed:8197120}; |
| Subellular location SUBCELLULAR LOCATION: [Botulinum neurotoxin type D]: Secreted {ECO:0000269|PubMed:11713244, ECO:0000269|PubMed:17581814, ECO:0000269|PubMed:2668193}.; SUBCELLULAR LOCATION: [Botulinum neurotoxin D light chain]: Secreted {ECO:0000269|PubMed:16252491, ECO:0000269|PubMed:8569530}. Note=In animals that have ingested BoNT/D LC acts in the eukaryotic host cytosol (Probable). {ECO:0000305|PubMed:15584922, ECO:0000305|PubMed:8175689}.; SUBCELLULAR LOCATION: [Botulinum neurotoxin D heavy chain]: Secreted {ECO:0000269|PubMed:16252491, ECO:0000269|PubMed:8569530}. Note=Upon incubation with hippocampal cell colocalizes with SV2C, probably in host synaptic vesicles (PubMed:21483489). Upon incubation with primary neurons HC colocalizes with synaptophysin probably in synaptic vesicles of presynaptic cells; a small portion also colocalizes with RAB5 and may be in synaptic vesicle protein sorting endosomes (PubMed:21632541). Probably integrates into the eukaryotic host synaptic vesicle membrane. {ECO:0000269|PubMed:21483489, ECO:0000269|PubMed:21632541, ECO:0000305}. |
| Structure SUBUNIT: Heterodimer; disulfide-linked heterodimer of a light chain (LC) and a heavy chain (HC) (PubMed:26324071). The LC has the proteolytic/pharmacological activity (PubMed:8175689, PubMed:8197120). The N- and C-termini of the HC mediate channel formation and eukaryotic host cell binding, respectively. Can also be purified in complex with a non-toxic component that is larger than the HC (PubMed:16252491). Single chain toxin from strain D-4947 copurifies with NTHNA, and in complexes that include NTNHA, HA-70, HA-33 and HA-17 (PubMed:11713244, PubMed:17581814). Dichain toxin from strain CB-16 phage d-16 phi copurifies with NTHNA, and in complexes that include NTNHA, HA-55, HA-33, HA-22 and HA-17 (PubMed:8569530). The stoichiometry of the whole complex has been modeled as one BoNT/D, one NTNHA, three HA-70, six HA-33 and three HA-17 (PubMed:17581814). HC interacts with eukaryotic protein synaptic vesicle glycoprotein 2B (SV2B), which may serve as its receptor (PubMed:21483489). Another group does not find a convincing interaction with SV2 (PubMed:21632541). {ECO:0000269|PubMed:11713244, ECO:0000269|PubMed:16252491, ECO:0000269|PubMed:16519520, ECO:0000269|PubMed:17581814, ECO:0000269|PubMed:21483489, ECO:0000269|PubMed:21632541, ECO:0000269|PubMed:8175689, ECO:0000269|PubMed:8197120, ECO:0000269|PubMed:8569530, ECO:0000305|PubMed:26324071}. |
| Domain DOMAIN: [Botulinum neurotoxin D light chain]: Has protease activity (PubMed:8175689, PubMed:8197120). {ECO:0000269|PubMed:8175689, ECO:0000269|PubMed:8197120}.; DOMAIN: [Botulinum neurotoxin D heavy chain]: Has 3 functional domains; the translocation domain (TD) and the receptor-binding domain (RBD) which is further subdivided into N- and C-terminal domains (N-RBD and C-RBD, also called Hcn and Hcc) (PubMed:20704566, PubMed:20731382). The N-terminus of the TD wraps an extended belt around the perimeter of the LC which occludes the catalytic pocket (PubMed:26324071). The belt region may be a pseudosubstrate inhibitor which serves as an intramolecular chaperone for the LC prior to its translocation into the host cytosol (PubMed:17907800). {ECO:0000269|PubMed:20704566, ECO:0000269|PubMed:20731382, ECO:0000269|PubMed:26324071, ECO:0000305|PubMed:17907800}. |
| Biotechnology BIOTECHNOLOGY: Cargo proteins fused to the N-terminus of whole toxin are imported into neurons; stabilized cargo proteins are poorer substrates, suggesting partial unfolding of the cargo protein is necessary for import (PubMed:15584922). {ECO:0000269|PubMed:15584922}.; BIOTECHNOLOGY: Can be used for targeted secretion inhibition in eukaryotic cells. A construct with a mammalian growth hormone-releasing factor ligand domain (GHRH) inserted between the LC and TD, when injected into rats, leads to decreased growth hormone production. The GHRH ligand domain binds to the GHRH receptor on somatotrophs where it is taken up into endosomes. There the TD inserts into the membrane, releasing LC which cleaves synaptobrevin and inhibits growth hormone exocytosis (PubMed:24029240, PubMed:26324071). {ECO:0000269|PubMed:24029240, ECO:0000305|PubMed:26324071}. |
| Target Relevance information above includes information from UniProt accession: P19321 |
| The UniProt Consortium |
Data
| No results found |
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| Western blot IHC ICC |
Documents
| # | SDS | Certificate | |
|---|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | |||
Only logged in customers who have purchased this product may leave a review.



Reviews
There are no reviews yet.