| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| target | Dengue Virus reactive IgG |
| species reactivity | Dengue Virus |
| applications | ELISA |
| assay type | Indirect & quantitative |
| available sizes | 96 tests |
Dengue Virus IgG ELISA Kit ESR114G
$364.00
Summary
- Virion/Serion Diagnostic Kit for research use (RUO)
- Dengue Virus (DENV) IgG ELISA Kit
- Suitable for IgG detection
- Ready-to-use
- 96 tests
Dengue Virus IgG ELISA Kit ESR114G
| kit | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay type Indirect ELISA | ||||||||||||||||||
| Research area Infectious Disease | ||||||||||||||||||
| Sample type Serum, plasma, whole blood | ||||||||||||||||||
Components
| ||||||||||||||||||
| Storage Store at 2-8°C. | ||||||||||||||||||
| Associated products Dengue Virus superior Human IgM Assay Control (BC1141M) Dengue Virus Human IgM Assay Control (BC114M) Dengue Virus Human IgG Assay Control (BC114G) Dengue Virus superior IgM ELISA Kit (ESR1141M) Dengue Virus IgG ELISA Kit (ESR114G) Dengue Virus IgM ELISA Kit (ESR114M) |
| target relevance |
|---|
| Organism Dengue Virus |
| Protein names Dengue Virus |
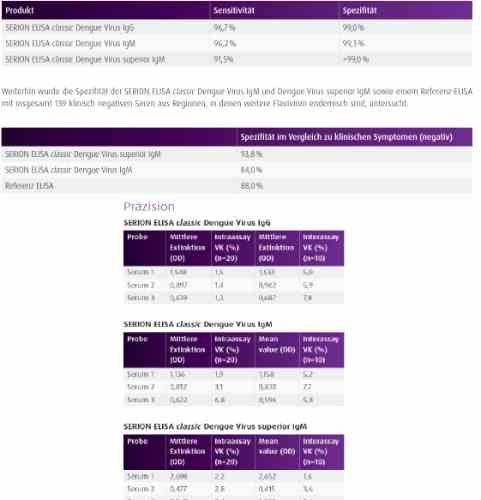
| Structure and strains Dengue virus (DENV) is the cause of dengue fever. It is a mosquito-borne, single positive-stranded RNA virus of the family Flaviviridae; genus Flavivirus. Four serotypes of the virus have been found, and a reported fifth has yet to be confirmed, all of which can cause the full spectrum of disease. Nevertheless, scientists' understanding of dengue virus may be simplistic as, rather than distinct antigenic groups, a continuum appears to exist. This same study identified 47 strains of dengue virus. Additionally, coinfection with and lack of rapid tests for Zika virus and chikungunya complicate matters in real-world infections. Dengue virus has increased dramatically within the last 20 years, becoming one of the worst mosquito-borne human pathogens that tropical countries have to deal with. Current estimates indicate that as many as 390 million infections occur each year, and many dengue infections are increasingly understood to be asymptomatic or subclinical. |
| Detection and diagnosis Between day 1 to 5 post onset of symptoms NS1 antigen detection as well as PCR are the most reliable methods to identify a Dengue Virus infection. Subsequently, serology is the method of choice for laboratory diagnostics. According to the Pan American Health Organization (PAHO) guidelines 80 % of all Dengue fever cases develop IgM antibodies by day 5 of illness, and 93-99 % have detectable IgM antibodies by day 6 post onset of symptoms, which may then remain detectable for more than 90 days. IgG antibodies are detectable at the end of the first week of illness and persist several months or even lifelong. |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| ESR114G protocol |
Documents
| # | ||
|---|---|---|
| EP0070 | Safety Data Sheet | QC certificate |
Only logged in customers who have purchased this product may leave a review.


Reviews
There are no reviews yet.