| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| host | rabbit |
| isotype | IgG |
| clonality | polyclonal |
| concentration | 0.2 mg/mL |
| applications | IP, WB |
| reactivity | human, mouse |
| available sizes | 10 µL, 100 µL |
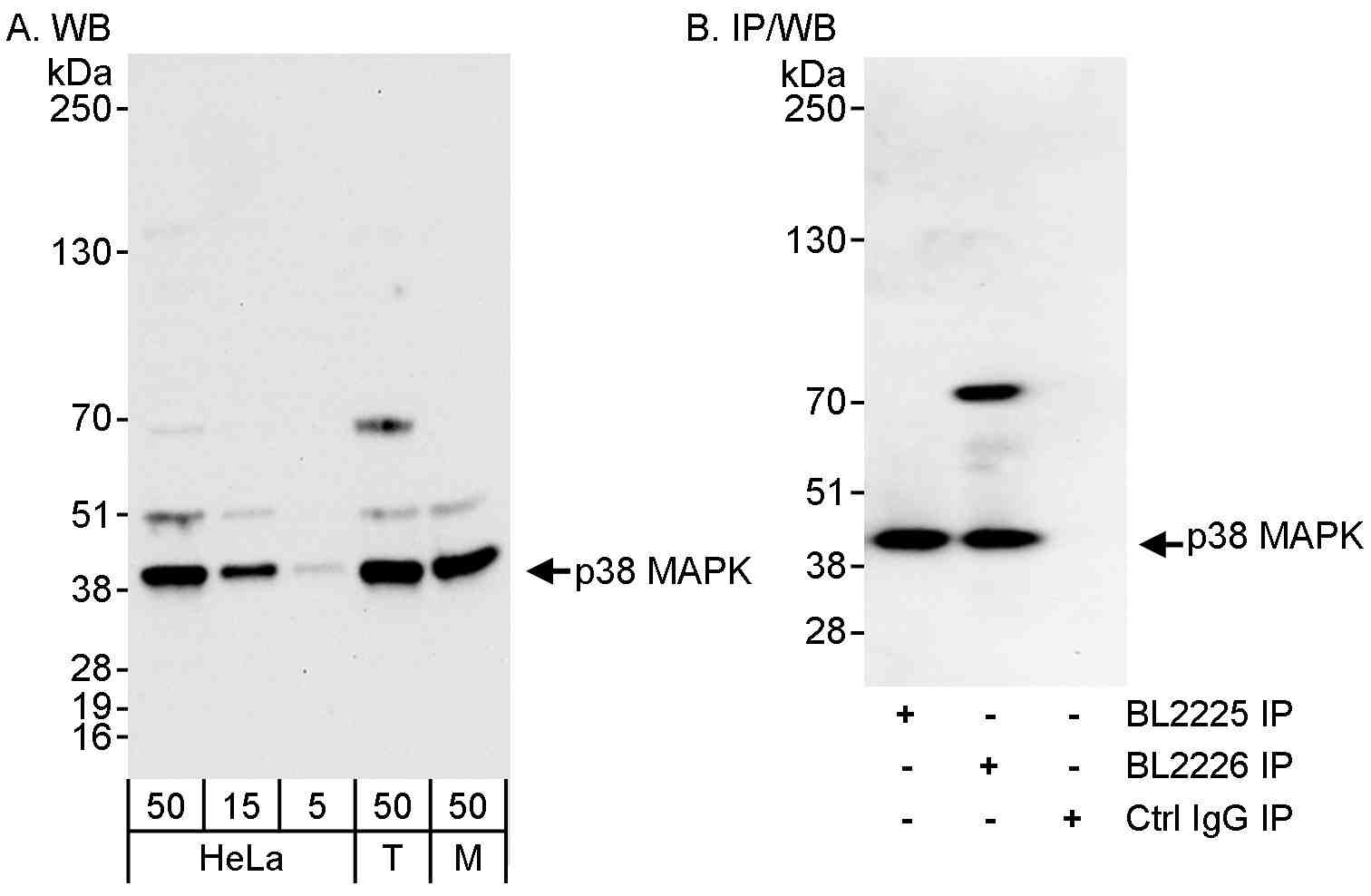
rabbit anti-p38 polyclonal antibody 1032
Price range: $100.00 through $2,600.00
Antibody summary
- Rabbit polyclonal to p38 MAPK
- Suitable for: IP,WB
- Reacts with: Hu, Ms presumed rat
- Isotype: IgG
- 100 µL, 20 µL
rabbit anti- p38 polyclonal antibody 1032
| target relevance |
|---|
| Protein names Mitogen-activated protein kinase 14 (MAP kinase 14) (MAPK 14) (EC 2.7.11.24) (Cytokine suppressive anti-inflammatory drug-binding protein) (CSAID-binding protein) (CSBP) (MAP kinase MXI2) (MAX-interacting protein 2) (Mitogen-activated protein kinase p38 alpha) (MAP kinase p38 alpha) (Stress-activated protein kinase 2a) (SAPK2a) |
| Gene names MAPK14,MAPK14 CSBP CSBP1 CSBP2 CSPB1 MXI2 SAPK2A |
| Protein family Protein kinase superfamily, CMGC Ser/Thr protein kinase family, MAP kinase subfamily |
| Mass 41293Da |
| Function FUNCTION: Serine/threonine kinase which acts as an essential component of the MAP kinase signal transduction pathway. MAPK14 is one of the four p38 MAPKs which play an important role in the cascades of cellular responses evoked by extracellular stimuli such as pro-inflammatory cytokines or physical stress leading to direct activation of transcription factors. Accordingly, p38 MAPKs phosphorylate a broad range of proteins and it has been estimated that they may have approximately 200 to 300 substrates each. Some of the targets are downstream kinases which are activated through phosphorylation and further phosphorylate additional targets. RPS6KA5/MSK1 and RPS6KA4/MSK2 can directly phosphorylate and activate transcription factors such as CREB1, ATF1, the NF-kappa-B isoform RELA/NFKB3, STAT1 and STAT3, but can also phosphorylate histone H3 and the nucleosomal protein HMGN1 (PubMed:9687510, PubMed:9792677). RPS6KA5/MSK1 and RPS6KA4/MSK2 play important roles in the rapid induction of immediate-early genes in response to stress or mitogenic stimuli, either by inducing chromatin remodeling or by recruiting the transcription machinery (PubMed:9687510, PubMed:9792677). On the other hand, two other kinase targets, MAPKAPK2/MK2 and MAPKAPK3/MK3, participate in the control of gene expression mostly at the post-transcriptional level, by phosphorylating ZFP36 (tristetraprolin) and ELAVL1, and by regulating EEF2K, which is important for the elongation of mRNA during translation. MKNK1/MNK1 and MKNK2/MNK2, two other kinases activated by p38 MAPKs, regulate protein synthesis by phosphorylating the initiation factor EIF4E2 (PubMed:11154262). MAPK14 also interacts with casein kinase II, leading to its activation through autophosphorylation and further phosphorylation of TP53/p53 (PubMed:10747897). In the cytoplasm, the p38 MAPK pathway is an important regulator of protein turnover. For example, CFLAR is an inhibitor of TNF-induced apoptosis whose proteasome-mediated degradation is regulated by p38 MAPK phosphorylation. In a similar way, MAPK14 phosphorylates the ubiquitin ligase SIAH2, regulating its activity towards EGLN3 (PubMed:17003045). MAPK14 may also inhibit the lysosomal degradation pathway of autophagy by interfering with the intracellular trafficking of the transmembrane protein ATG9 (PubMed:19893488). Another function of MAPK14 is to regulate the endocytosis of membrane receptors by different mechanisms that impinge on the small GTPase RAB5A. In addition, clathrin-mediated EGFR internalization induced by inflammatory cytokines and UV irradiation depends on MAPK14-mediated phosphorylation of EGFR itself as well as of RAB5A effectors (PubMed:16932740). Ectodomain shedding of transmembrane proteins is regulated by p38 MAPKs as well. In response to inflammatory stimuli, p38 MAPKs phosphorylate the membrane-associated metalloprotease ADAM17 (PubMed:20188673). Such phosphorylation is required for ADAM17-mediated ectodomain shedding of TGF-alpha family ligands, which results in the activation of EGFR signaling and cell proliferation. Another p38 MAPK substrate is FGFR1. FGFR1 can be translocated from the extracellular space into the cytosol and nucleus of target cells, and regulates processes such as rRNA synthesis and cell growth. FGFR1 translocation requires p38 MAPK activation. In the nucleus, many transcription factors are phosphorylated and activated by p38 MAPKs in response to different stimuli. Classical examples include ATF1, ATF2, ATF6, ELK1, PTPRH, DDIT3, TP53/p53 and MEF2C and MEF2A (PubMed:10330143, PubMed:9430721, PubMed:9858528). The p38 MAPKs are emerging as important modulators of gene expression by regulating chromatin modifiers and remodelers. The promoters of several genes involved in the inflammatory response, such as IL6, IL8 and IL12B, display a p38 MAPK-dependent enrichment of histone H3 phosphorylation on 'Ser-10' (H3S10ph) in LPS-stimulated myeloid cells. This phosphorylation enhances the accessibility of the cryptic NF-kappa-B-binding sites marking promoters for increased NF-kappa-B recruitment. Phosphorylates CDC25B and CDC25C which is required for binding to 14-3-3 proteins and leads to initiation of a G2 delay after ultraviolet radiation (PubMed:11333986). Phosphorylates TIAR following DNA damage, releasing TIAR from GADD45A mRNA and preventing mRNA degradation (PubMed:20932473). The p38 MAPKs may also have kinase-independent roles, which are thought to be due to the binding to targets in the absence of phosphorylation. Protein O-Glc-N-acylation catalyzed by the OGT is regulated by MAPK14, and, although OGT does not seem to be phosphorylated by MAPK14, their interaction increases upon MAPK14 activation induced by glucose deprivation. This interaction may regulate OGT activity by recruiting it to specific targets such as neurofilament H, stimulating its O-Glc-N-acylation. Required in mid-fetal development for the growth of embryo-derived blood vessels in the labyrinth layer of the placenta. Also plays an essential role in developmental and stress-induced erythropoiesis, through regulation of EPO gene expression (PubMed:10943842). Isoform MXI2 activation is stimulated by mitogens and oxidative stress and only poorly phosphorylates ELK1 and ATF2. Isoform EXIP may play a role in the early onset of apoptosis. Phosphorylates S100A9 at 'Thr-113' (PubMed:15905572). Phosphorylates NLRP1 downstream of MAP3K20/ZAK in response to UV-B irradiation and ribosome collisions, promoting activation of the NLRP1 inflammasome and pyroptosis (PubMed:35857590). {ECO:0000269|PubMed:10330143, ECO:0000269|PubMed:10747897, ECO:0000269|PubMed:10943842, ECO:0000269|PubMed:11154262, ECO:0000269|PubMed:11333986, ECO:0000269|PubMed:15905572, ECO:0000269|PubMed:16932740, ECO:0000269|PubMed:17003045, ECO:0000269|PubMed:17724032, ECO:0000269|PubMed:19893488, ECO:0000269|PubMed:20188673, ECO:0000269|PubMed:20932473, ECO:0000269|PubMed:35857590, ECO:0000269|PubMed:9430721, ECO:0000269|PubMed:9687510, ECO:0000269|PubMed:9792677, ECO:0000269|PubMed:9858528}.; FUNCTION: (Microbial infection) Activated by phosphorylation by M.tuberculosis EsxA in T-cells leading to inhibition of IFN-gamma production; phosphorylation is apparent within 15 minutes and is inhibited by kinase-specific inhibitors SB203580 and siRNA (PubMed:21586573). {ECO:0000269|PubMed:21586573}. |
| Catalytic activity CATALYTIC ACTIVITY: Reaction=L-seryl-[protein] + ATP = O-phospho-L-seryl-[protein] + ADP + H(+); Xref=Rhea:RHEA:17989, Rhea:RHEA-COMP:9863, Rhea:RHEA-COMP:11604, ChEBI:CHEBI:15378, ChEBI:CHEBI:29999, ChEBI:CHEBI:30616, ChEBI:CHEBI:83421, ChEBI:CHEBI:456216; EC=2.7.11.24; Evidence={ECO:0000269|PubMed:11010976, ECO:0000269|PubMed:35857590}; PhysiologicalDirection=left-to-right; Xref=Rhea:RHEA:17990; Evidence={ECO:0000269|PubMed:11010976, ECO:0000269|PubMed:35857590}; CATALYTIC ACTIVITY: Reaction=L-threonyl-[protein] + ATP = O-phospho-L-threonyl-[protein] + ADP + H(+); Xref=Rhea:RHEA:46608, Rhea:RHEA-COMP:11060, Rhea:RHEA-COMP:11605, ChEBI:CHEBI:15378, ChEBI:CHEBI:30013, ChEBI:CHEBI:30616, ChEBI:CHEBI:61977, ChEBI:CHEBI:456216; EC=2.7.11.24; Evidence={ECO:0000269|PubMed:11010976, ECO:0000269|PubMed:35857590}; PhysiologicalDirection=left-to-right; Xref=Rhea:RHEA:46609; Evidence={ECO:0000269|PubMed:11010976, ECO:0000269|PubMed:35857590}; |
| Subellular location SUBCELLULAR LOCATION: Cytoplasm {ECO:0000269|PubMed:7535770}. Nucleus {ECO:0000269|PubMed:30878395, ECO:0000269|PubMed:7535770}. |
| Tissues TISSUE SPECIFICITY: Brain, heart, placenta, pancreas and skeletal muscle. Expressed to a lesser extent in lung, liver and kidney. |
| Structure SUBUNIT: Component of a signaling complex containing at least AKAP13, PKN1, MAPK14, ZAK and MAP2K3. Within this complex, AKAP13 interacts directly with PKN1, which in turn recruits MAPK14, MAP2K3 and ZAK (PubMed:21224381). Binds to a kinase interaction motif within the protein tyrosine phosphatase, PTPRR (By similarity). This interaction retains MAPK14 in the cytoplasm and prevents nuclear accumulation (By similarity). Interacts with SPAG9 and GADD45A (By similarity). Interacts with CDC25B, CDC25C, DUSP1, DUSP10, DUSP16, NP60, SUPT20H and TAB1. Interacts with casein kinase II subunits CSNK2A1 and CSNK2B. Interacts with PPM1D. Interacts with CDK5RAP3; recruits PPM1D to MAPK14 and may regulate its dephosphorylation (PubMed:21283629). Interacts with DUSP2; this interaction does not lead to catalytic activation of DUSP2 and dephosphrylation of MAPK14 (By similarity). {ECO:0000250|UniProtKB:P47811, ECO:0000269|PubMed:10391943, ECO:0000269|PubMed:10747897, ECO:0000269|PubMed:11010976, ECO:0000269|PubMed:11278799, ECO:0000269|PubMed:11333986, ECO:0000269|PubMed:11359773, ECO:0000269|PubMed:11847341, ECO:0000269|PubMed:12897767, ECO:0000269|PubMed:15658854, ECO:0000269|PubMed:15735649, ECO:0000269|PubMed:16342939, ECO:0000269|PubMed:16352664, ECO:0000269|PubMed:16751104, ECO:0000269|PubMed:17255097, ECO:0000269|PubMed:20188673, ECO:0000269|PubMed:21224381, ECO:0000269|PubMed:21283629, ECO:0000269|PubMed:9792677}. |
| Post-translational modification PTM: Dually phosphorylated on Thr-180 and Tyr-182 by the MAP2Ks MAP2K3/MKK3, MAP2K4/MKK4 and MAP2K6/MKK6 in response to inflammatory citokines, environmental stress or growth factors, which activates the enzyme. Dual phosphorylation can also be mediated by TAB1-mediated autophosphorylation. TCR engagement in T-cells also leads to Tyr-323 phosphorylation by ZAP70. Dephosphorylated and inactivated by DUPS1, DUSP10 and DUSP16. PPM1D also mediates dephosphorylation and inactivation of MAPK14 (PubMed:21283629). {ECO:0000269|PubMed:11010976, ECO:0000269|PubMed:15735648, ECO:0000269|PubMed:17724032, ECO:0000269|PubMed:21283629, ECO:0000269|PubMed:7535770, ECO:0000269|PubMed:8622669}.; PTM: Acetylated at Lys-53 and Lys-152 by KAT2B and EP300. Acetylation at Lys-53 increases the affinity for ATP and enhances kinase activity. Lys-53 and Lys-152 are deacetylated by HDAC3. {ECO:0000269|PubMed:21444723}.; PTM: Ubiquitinated. Ubiquitination leads to degradation by the proteasome pathway. {ECO:0000269|PubMed:17724032}. |
| Domain DOMAIN: The TXY motif contains the threonine and tyrosine residues whose phosphorylation activates the MAP kinases. |
| Target Relevance information above includes information from UniProt accession: Q16539 |
| The UniProt Consortium |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| Western blot |
Documents
| # | SDS | Certificate | |
|---|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | |||
Only logged in customers who have purchased this product may leave a review.

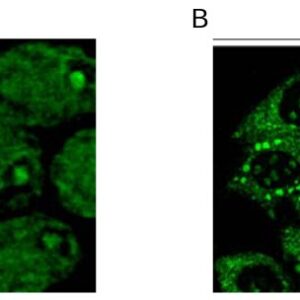
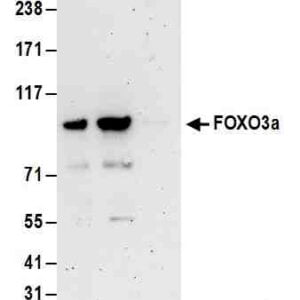
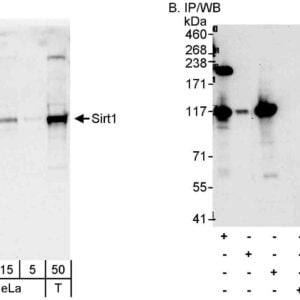
Reviews
There are no reviews yet.