| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| host | mouse |
| isotype | IgG1 |
| clonality | monoclonal |
| concentration | 1 mg/mL |
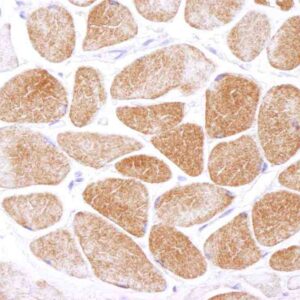
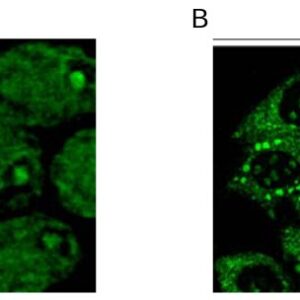
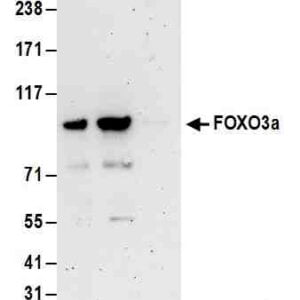
| applications | ICC/IF, IHC, WB |
| available sizes | 100 µg |
mouse anti-Alpha-synuclein monoclonal antibody (211) 9067
$409.00
Antibody summary
- Mouse monoclonal to Alpha-synuclein
- Suitable for: WB, ICC/IF, IHC
- Reacts with: human, mouse, rat
- Isotype: IgG1
- 100 µg
mouse anti-Alpha-synuclein monoclonal antibody (211) 9067
| target relevance |
|---|
| Alpha-synuclein is a crucial protein involved in synaptic function and neurotransmitter release, primarily found in presynaptic terminals of neurons. In research, alpha-synuclein has gained significant attention as a potential cell marker for neurodegenerative diseases, particularly Parkinson's disease. The aggregation of alpha-synuclein into insoluble fibrils is a hallmark of Parkinson's pathology, and its presence in Lewy bodies is a diagnostic feature of the disease. Therefore, alpha-synuclein is utilized as a marker to identify and study Parkinson's disease-related pathology in postmortem brain tissues and experimental models. Antibodies targeting alpha-synuclein are extensively employed in immunohistochemistry and western blotting to visualize and quantify its expression levels in various brain regions and cellular compartments. Click for more on: cell markers and alpha synuclein |
| Protein names Alpha-synuclein (Non-A beta component of AD amyloid) (Non-A4 component of amyloid precursor) (NACP) |
| Gene names SNCA,SNCA NACP PARK1 |
| Protein family Synuclein family |
| Mass 14460Da |
| Function FUNCTION: Neuronal protein that plays several roles in synaptic activity such as regulation of synaptic vesicle trafficking and subsequent neurotransmitter release (PubMed:20798282, PubMed:26442590, PubMed:28288128, PubMed:30404828). Participates as a monomer in synaptic vesicle exocytosis by enhancing vesicle priming, fusion and dilation of exocytotic fusion pores (PubMed:28288128, PubMed:30404828). Mechanistically, acts by increasing local Ca(2+) release from microdomains which is essential for the enhancement of ATP-induced exocytosis (PubMed:30404828). Also acts as a molecular chaperone in its multimeric membrane-bound state, assisting in the folding of synaptic fusion components called SNAREs (Soluble NSF Attachment Protein REceptors) at presynaptic plasma membrane in conjunction with cysteine string protein-alpha/DNAJC5 (PubMed:20798282). This chaperone activity is important to sustain normal SNARE-complex assembly during aging (PubMed:20798282). Also plays a role in the regulation of the dopamine neurotransmission by associating with the dopamine transporter (DAT1) and thereby modulating its activity (PubMed:26442590). {ECO:0000269|PubMed:20798282, ECO:0000269|PubMed:26442590, ECO:0000269|PubMed:28288128, ECO:0000269|PubMed:30404828}. |
| Subellular location SUBCELLULAR LOCATION: Cytoplasm {ECO:0000269|PubMed:19762560, ECO:0000269|PubMed:24936070, ECO:0000269|PubMed:25561023, ECO:0000269|PubMed:26442590, ECO:0000269|PubMed:31034892, ECO:0000269|PubMed:33657088}. Membrane {ECO:0000269|PubMed:24936070}. Nucleus {ECO:0000269|PubMed:12859192, ECO:0000269|PubMed:24936070}. Synapse {ECO:0000269|PubMed:15282274}. Secreted {ECO:0000269|PubMed:24936070}. Cell projection, axon {ECO:0000250|UniProtKB:O55042}. Note=Membrane-bound in dopaminergic neurons (PubMed:15282274). Expressed and colocalized with SEPTIN4 in dopaminergic axon terminals, especially at the varicosities (By similarity). {ECO:0000250|UniProtKB:O55042, ECO:0000269|PubMed:15282274}. |
| Tissues TISSUE SPECIFICITY: Highly expressed in presynaptic terminals in the central nervous system. Expressed principally in brain. {ECO:0000269|PubMed:8194594}. |
| Structure SUBUNIT: Soluble monomer. Homotetramer (PubMed:21841800). A dynamic intracellular population of tetramers and monomers coexists normally and the tetramer plays an essential role in maintaining homeostasis (PubMed:21841800). Interacts with UCHL1 (By similarity). Interacts with phospholipase D and histones. Interacts (via N-terminus) with synphilin-1/SNCAIP; this interaction promotes formation of SNCA inclusions in the cytoplasm (PubMed:19762560). Interacts with CALM1 (PubMed:23607618). Interacts with STXBP1; this interaction controls SNCA self-replicating aggregation (PubMed:27597756). Interacts with SNARE components VAMP2 and SNAP25; these interactions allows SNARE complex assembly and integrity (PubMed:20798282). Interacts with RPH3A and RAB3A (PubMed:15207266). Interacts with SERF1A; this interaction promotes the aggregation of SNCA (PubMed:22854022, PubMed:31034892). Interacts with SEPTIN4 (By similarity). Interacts with DDX10; this interaction causes DDX10 mislocalization to the nucleoplasm and cytoplasmic inclusions (PubMed:33657088). {ECO:0000250|UniProtKB:O55042, ECO:0000250|UniProtKB:P37377, ECO:0000269|PubMed:15207266, ECO:0000269|PubMed:19762560, ECO:0000269|PubMed:20798282, ECO:0000269|PubMed:21841800, ECO:0000269|PubMed:22854022, ECO:0000269|PubMed:23607618, ECO:0000269|PubMed:27597756, ECO:0000269|PubMed:31034892, ECO:0000269|PubMed:33657088}. |
| Post-translational modification PTM: Phosphorylated, predominantly on serine residues. Phosphorylation by CK1 appears to occur on residues distinct from the residue phosphorylated by other kinases. Phosphorylation of Ser-129 is selective and extensive in synucleinopathy lesions. In vitro, phosphorylation at Ser-129 promoted insoluble fibril formation. Phosphorylated on Tyr-125 by a PTK2B-dependent pathway upon osmotic stress. {ECO:0000269|PubMed:10617630, ECO:0000269|PubMed:10852916, ECO:0000269|PubMed:11162638, ECO:0000269|PubMed:11813001, ECO:0000269|PubMed:12893833, ECO:0000269|PubMed:24936070}.; PTM: Hallmark lesions of neurodegenerative synucleinopathies contain alpha-synuclein that is modified by nitration of tyrosine residues and possibly by dityrosine cross-linking to generated stable oligomers.; PTM: Ubiquitinated. The predominant conjugate is the diubiquitinated form. {ECO:0000250|UniProtKB:P37377}.; PTM: Acetylation at Met-1 seems to be important for proper folding and native oligomeric structure. {ECO:0000269|PubMed:22407793}. |
| Domain DOMAIN: The 'non A-beta component of Alzheimer disease amyloid plaque' domain (NAC domain) is involved in fibrils formation. The middle hydrophobic region forms the core of the filaments. The C-terminus may regulate aggregation and determine the diameter of the filaments. {ECO:0000269|PubMed:19722699}. |
| Involvement in disease DISEASE: Note=Genetic alterations of SNCA resulting in aberrant polymerization into fibrils, are associated with several neurodegenerative diseases (synucleinopathies). SNCA fibrillar aggregates represent the major non A-beta component of Alzheimer disease amyloid plaque, and a major component of Lewy body inclusions. They are also found within Lewy body (LB)-like intraneuronal inclusions, glial inclusions and axonal spheroids in neurodegeneration with brain iron accumulation type 1.; DISEASE: Parkinson disease 1, autosomal dominant (PARK1) [MIM:168601]: A complex neurodegenerative disorder characterized by bradykinesia, resting tremor, muscular rigidity and postural instability. Additional features are characteristic postural abnormalities, dysautonomia, dystonic cramps, and dementia. The pathology of Parkinson disease involves the loss of dopaminergic neurons in the substantia nigra and the presence of Lewy bodies (intraneuronal accumulations of aggregated proteins), in surviving neurons in various areas of the brain. The disease is progressive and usually manifests after the age of 50 years, although early-onset cases (before 50 years) are known. The majority of the cases are sporadic suggesting a multifactorial etiology based on environmental and genetic factors. However, some patients present with a positive family history for the disease. Familial forms of the disease usually begin at earlier ages and are associated with atypical clinical features. {ECO:0000269|PubMed:14755719, ECO:0000269|PubMed:23427326, ECO:0000269|PubMed:23457019, ECO:0000269|PubMed:24936070, ECO:0000269|PubMed:25561023, ECO:0000269|PubMed:9197268, ECO:0000269|PubMed:9462735}. Note=The disease is caused by variants affecting the gene represented in this entry.; DISEASE: Parkinson disease 4, autosomal dominant (PARK4) [MIM:605543]: A complex neurodegenerative disorder with manifestations ranging from typical Parkinson disease to dementia with Lewy bodies. Clinical features include parkinsonian symptoms (resting tremor, rigidity, postural instability and bradykinesia), dementia, diffuse Lewy body pathology, autonomic dysfunction, hallucinations and paranoia. Note=The disease is caused by variants affecting the gene represented in this entry.; DISEASE: Dementia, Lewy body (DLB) [MIM:127750]: A neurodegenerative disorder characterized by mental impairment leading to dementia, parkinsonism, fluctuating cognitive function, visual hallucinations, falls, syncopal episodes, and sensitivity to neuroleptic medication. Brainstem or cortical intraneuronal accumulations of aggregated proteins (Lewy bodies) are the only essential pathologic features. Patients may also have hippocampal and neocortical senile plaques, sometimes in sufficient number to fulfill the diagnostic criteria for Alzheimer disease. {ECO:0000269|PubMed:14755719}. Note=The disease is caused by variants affecting the gene represented in this entry. |
| Target Relevance information above includes information from UniProt accession: P37840 |
| The UniProt Consortium |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| Western blot IHC ICC |
Documents
| # | SDS | Certificate | |
|---|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | |||
Only logged in customers who have purchased this product may leave a review.






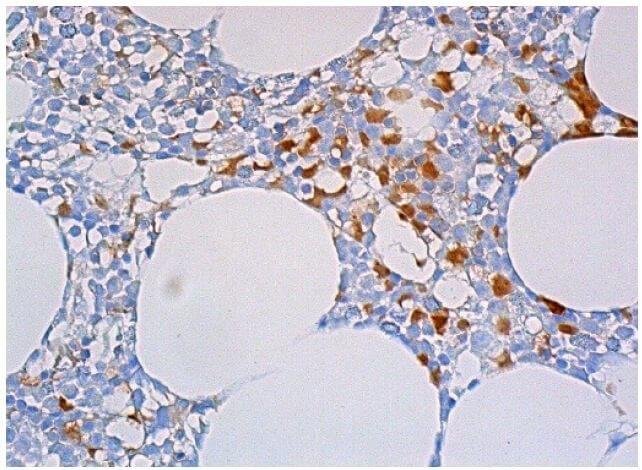


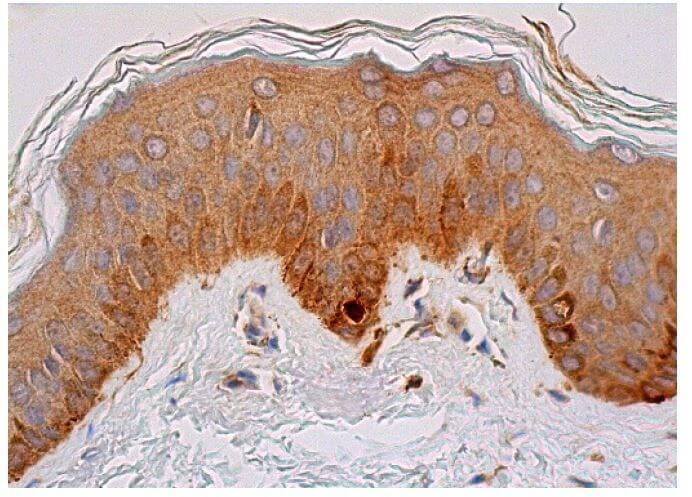
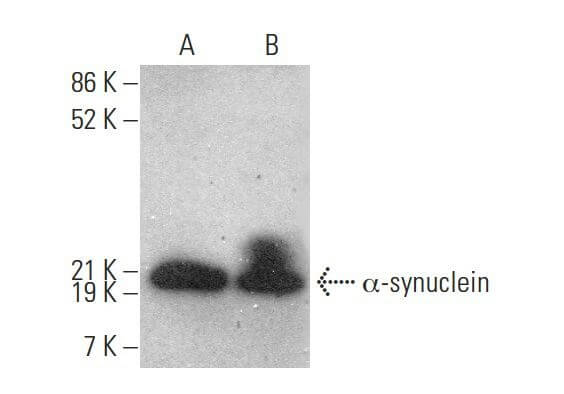
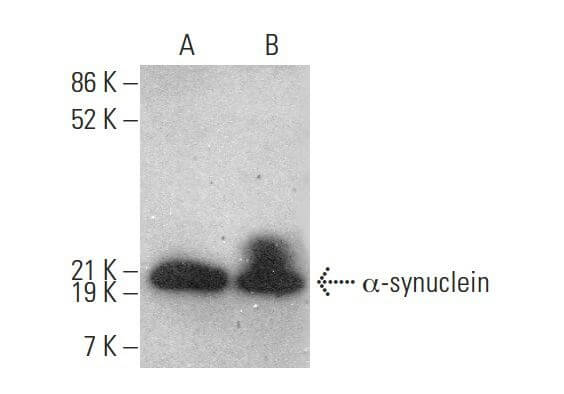
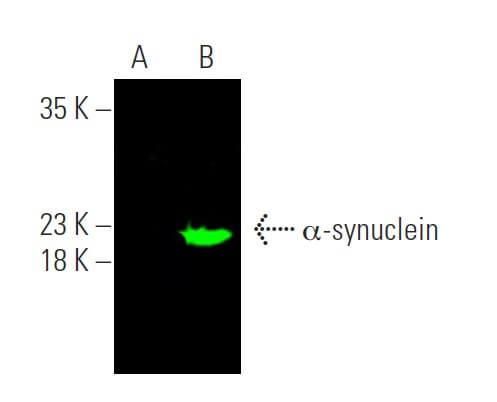
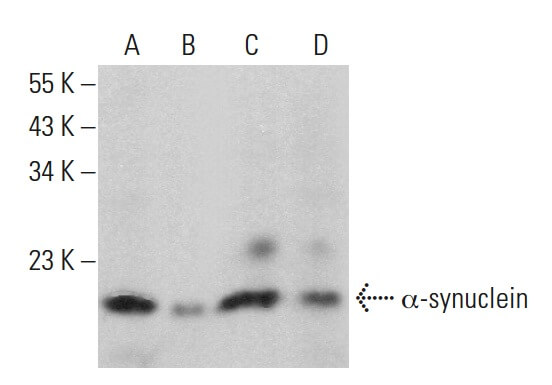

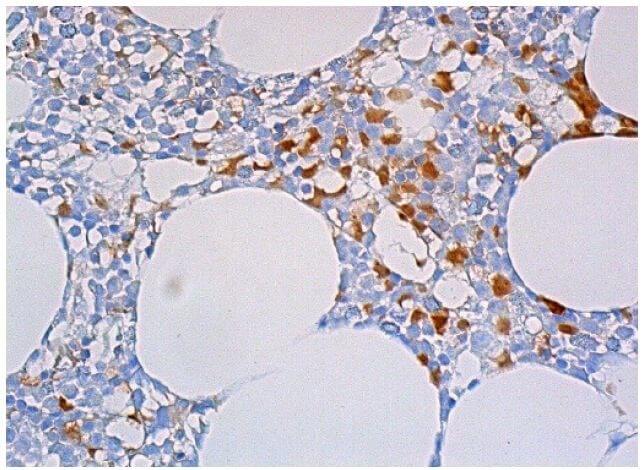



Reviews
There are no reviews yet.