| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| host | mouse |
| isotype | IgG1 |
| clonality | monoclonal |
| concentration | 1 mg/mL |
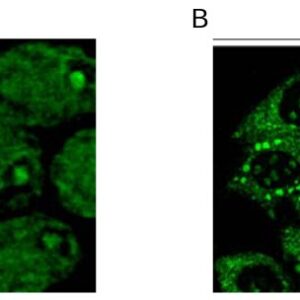
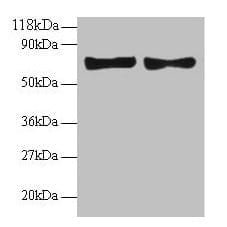
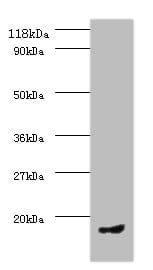
| applications | ICC/IF, WB |
| reactivity | cdc34 |
| available sizes | 100 µg |
mouse anti-cdc34 monoclonal antibody (14B5) 7470
$503.00
Antibody summary
- Mouse monoclonal to cdc34
- Suitable for: WB,ICC/IF,IHC-P,IP
- Isotype: IgG1
- 100 µg
mouse anti-cdc34 monoclonal antibody (14B5) 7470
| antibody |
|---|
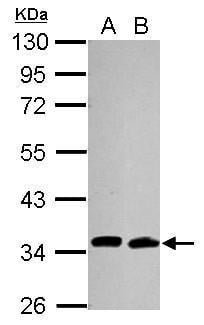
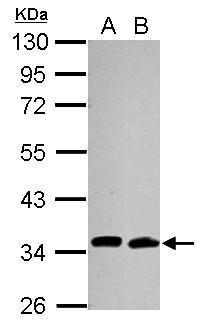
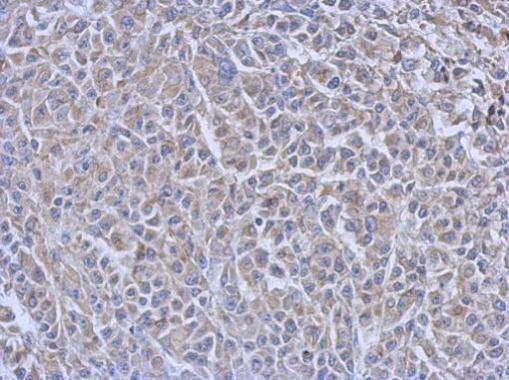
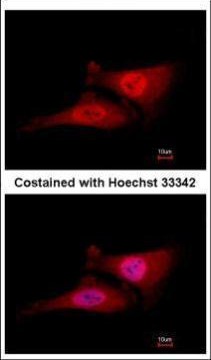
| Tested applications WB,IHC,IHC,ICC/IF |
| Recommended dilutions Immunoblotting and Immuno-precipitation: use at 1-5 ug/mL. Positive controls: 293 cells and recombinant fusion protein. |
| Immunogen N-terminus 6x-His tagged fusion protein encoding full-length human Cdc34 expressed in E. coli. |
| Size and concentration 100µg and lot specific |
| Form liquid |
| Storage Instructions This antibody is stable for at least one (1) year at -20°C. Avoid multiple freeze- thaw cycles. |
| Storage buffer 10 mM PBS, pH 7.4. |
| Purity immunogen affinity purification |
| Clonality monoclonal |
| Isotype IgG1 |
| Compatible secondaries goat anti-mouse IgG, H&L chain specific, peroxidase conjugated polyclonal antibody 5486 goat anti-mouse IgG, H&L chain specific, biotin conjugated, Conjugate polyclonal antibody 2685 goat anti-mouse IgG, H&L chain specific, FITC conjugated polyclonal antibody 7854 goat anti-mouse IgG, H&L chain specific, peroxidase conjugated polyclonal antibody, crossabsorbed 1706 goat anti-mouse IgG, H&L chain specific, biotin conjugated polyclonal antibody, crossabsorbed 1716 goat anti-mouse IgG, H&L chain specific, FITC conjugated polyclonal antibody, crossabsorbed 1721 |
| Isotype control Mouse monocolonal IgG1 - Isotype Control |
| target relevance |
|---|
| Protein names Ubiquitin-conjugating enzyme E2 R1 (EC 2.3.2.23) ((E3-independent) E2 ubiquitin-conjugating enzyme R1) (EC 2.3.2.24) (E2 ubiquitin-conjugating enzyme R1) (Ubiquitin-conjugating enzyme E2-32 kDa complementing) (Ubiquitin-conjugating enzyme E2-CDC34) (Ubiquitin-protein ligase R1) |
| Gene names CDC34,CDC34 UBCH3 UBE2R1 |
| Protein family Ubiquitin-conjugating enzyme family |
| Mass 26737Da |
| Function FUNCTION: E2 ubiquitin-conjugating enzyme that accepts ubiquitin from an E1 ubiquitin-activating protein, and catalyzes its covalent attachment to other proteins by an E3 ubiquitin-protein ligase complex (PubMed:10329681, PubMed:17588522, PubMed:20061386, PubMed:38326650). In vitro catalyzes 'Lys-48'-linked polyubiquitination (PubMed:22496338). Cooperates with the E2 UBCH5C and the SCF(FBXW11) E3 ligase complex for the polyubiquitination of NFKBIA leading to its subsequent proteasomal degradation (PubMed:10329681, PubMed:10918611, PubMed:17698585). Performs ubiquitin chain elongation building ubiquitin chains from the UBE2D3-primed NFKBIA-linked ubiquitin. UBE2D3 acts as an initiator E2, priming the phosphorylated NFKBIA target at positions 'Lys-21' and/or 'Lys-22' with a monoubiquitin. Cooperates with the SCF(SKP2) E3 ligase complex to regulate cell proliferation through ubiquitination and degradation of MYBL2 and KIP1 (PubMed:10871850, PubMed:15652359, PubMed:19112177). Involved in ubiquitin conjugation and degradation of CREM isoform ICERIIgamma and ATF15 resulting in abrogation of ICERIIgamma- and ATF5-mediated repression of cAMP-induced transcription during both meiotic and mitotic cell cycles. Involved in the regulation of the cell cycle G2/M phase through its targeting of the WEE1 kinase for ubiquitination and degradation (PubMed:19126550). Also involved in the degradation of beta-catenin (PubMed:12037680). Is target of human herpes virus 1 protein ICP0, leading to ICP0-dependent dynamic interaction with proteasomes (PubMed:11805320, PubMed:12060736). {ECO:0000269|PubMed:10329681, ECO:0000269|PubMed:10871850, ECO:0000269|PubMed:10918611, ECO:0000269|PubMed:11805320, ECO:0000269|PubMed:12037680, ECO:0000269|PubMed:12060736, ECO:0000269|PubMed:15652359, ECO:0000269|PubMed:17588522, ECO:0000269|PubMed:17698585, ECO:0000269|PubMed:19112177, ECO:0000269|PubMed:19126550, ECO:0000269|PubMed:20061386, ECO:0000269|PubMed:22496338, ECO:0000269|PubMed:38326650}. |
| Catalytic activity CATALYTIC ACTIVITY: Reaction=S-ubiquitinyl-[E1 ubiquitin-activating enzyme]-L-cysteine + [E2 ubiquitin-conjugating enzyme]-L-cysteine = [E1 ubiquitin-activating enzyme]-L-cysteine + S-ubiquitinyl-[E2 ubiquitin-conjugating enzyme]-L-cysteine.; EC=2.3.2.23; Evidence={ECO:0000255|PROSITE-ProRule:PRU00388, ECO:0000255|PROSITE-ProRule:PRU10133, ECO:0000269|PubMed:12037680, ECO:0000269|PubMed:17698585, ECO:0000269|PubMed:20061386, ECO:0000269|PubMed:20347421, ECO:0000269|PubMed:38326650}; CATALYTIC ACTIVITY: Reaction=S-ubiquitinyl-[E1 ubiquitin-activating enzyme]-L-cysteine + [acceptor protein]-L-lysine = [E1 ubiquitin-activating enzyme]-L-cysteine + N(6)-monoubiquitinyl-[acceptor protein]-L-lysine.; EC=2.3.2.24; Evidence={ECO:0000269|PubMed:17588522}; |
| Pathway PATHWAY: Protein modification; protein ubiquitination. {ECO:0000269|PubMed:38326650}. |
| Subellular location SUBCELLULAR LOCATION: Cytoplasm. Nucleus. Note=The phosphorylation of the C-terminal tail plays an important role in mediating nuclear localization. Colocalizes with beta-tubulin on mitotic spindles in anaphase. |
| Tissues TISSUE SPECIFICITY: Expressed in testes during spermatogenesis to regulate repression of cAMP-induced transcription. {ECO:0000269|PubMed:10373550}. |
| Structure SUBUNIT: Interacts with multiple Cul1-RING E3 ubiquitin-protein ligase complexes, also known as SCF (SKP1-CUL1-F-box protein) complexes (PubMed:10918611, PubMed:11675391, PubMed:18851830, PubMed:19112177, PubMed:19945379, PubMed:24316736). Identified in a SCF E3 ubiquitin ligase complex together with HINT1 and RBX1 (PubMed:19112177). When cullin is neddylated, the interaction between the E2 and the SCF complex is strengthened (PubMed:10918611, PubMed:11675391, PubMed:18851830, PubMed:19112177, PubMed:19945379, PubMed:24316736). Interacts with multiple Cul2-RING (CRL2) E3 ubiquitin-protein ligase complexes, also known as ECS (Elongin BC-CUL2/5-SOCS-box protein) complexes (PubMed:38326650). When phosphorylated, interacts with beta-TrCP (BTRC) (PubMed:12037680). Interacts with human herpes virus 1 protein ICP0 and associates with the proteasome for degradation (PubMed:11447293, PubMed:11805320, PubMed:12060736). Interacts with casein kinase subunit CSNK2B (PubMed:11546811). Interacts with CNTD1; this interaction regulates the cell-cycle progression (By similarity). {ECO:0000250|UniProtKB:Q8CFI2, ECO:0000269|PubMed:10918611, ECO:0000269|PubMed:11447293, ECO:0000269|PubMed:11546811, ECO:0000269|PubMed:11675391, ECO:0000269|PubMed:11805320, ECO:0000269|PubMed:12037680, ECO:0000269|PubMed:12060736, ECO:0000269|PubMed:18851830, ECO:0000269|PubMed:19112177, ECO:0000269|PubMed:19945379, ECO:0000269|PubMed:24316736}. |
| Post-translational modification PTM: Autoubiquitinated (PubMed:11805320, PubMed:12060736, PubMed:22496338). Autoubiquitination is promoted by the human herpes virus 1 protein ICP0 and leads to degradation by the Ubiquitin-proteasomal pathway (PubMed:11805320, PubMed:12060736). {ECO:0000269|PubMed:11805320, ECO:0000269|PubMed:12060736, ECO:0000269|PubMed:22496338}.; PTM: Phosphorylated by CK2. Phosphorylation of the C-terminal tail by CK2 controls the nuclear localization. {ECO:0000269|PubMed:11546811, ECO:0000269|PubMed:12037680, ECO:0000269|PubMed:17461777}. |
| Domain DOMAIN: The C-terminal acidic tail is required for nuclear localization and is involved in the binding to SCF E3 ligase complexes, and more specifically with the CUL1 subunit. {ECO:0000269|PubMed:10769200, ECO:0000269|PubMed:19945379}. |
| Target Relevance information above includes information from UniProt accession: P49427 |
| The UniProt Consortium |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| Western blot IHC ICC |
Documents
| # | SDS | Certificate | |
|---|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | |||
Only logged in customers who have purchased this product may leave a review.





Reviews
There are no reviews yet.