| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| target | Human Measles Virus reactive IgG |
| species reactivity | Measles Virus |
| applications | ELISA |
| assay type | Indirect & quantitative |
| available sizes | 96 tests |
Measles Virus IgG ELISA Kit ESR102G
$364.00
Summary
- Virion/Serion Diagnostic Kit for research use (RUO)
- Measles Virus IgG ELISA Kit
- Suitable for IgG detection
- Ready-to-use
- 96 tests
Measles Virus IgG ELISA Kit ESR102G
| kit | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target name Human Measles Virus IgG | ||||||||||||||||||
| Assay type Indirect ELISA | ||||||||||||||||||
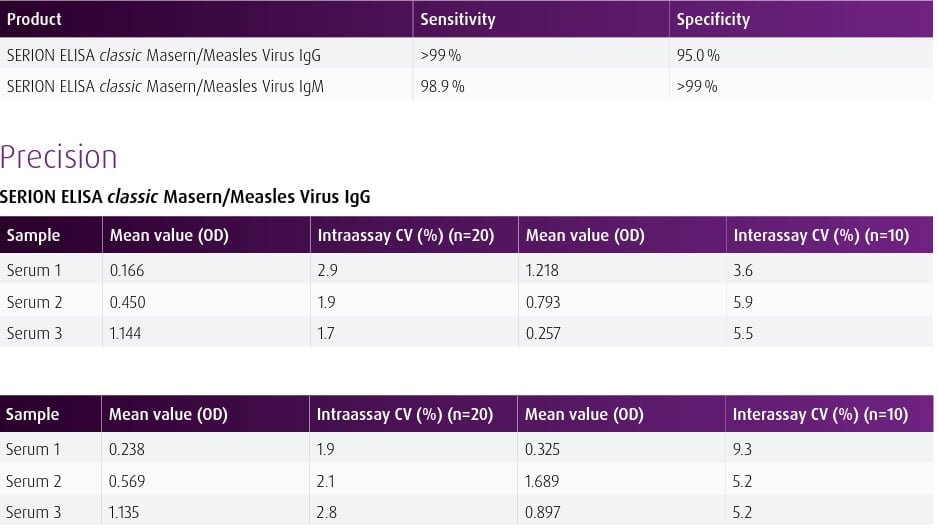
| Overview The SERION ELISA classic Masern/Measles Virus IgG and IgM tests are quantitative and qualitative immunoassays for the detection of human antibodies in serum or plasma directed against Measles Virus. The SERION ELISA classic Masern/Measles Virus IgM test is recommended for the determination of acute infections. The SERION ELISA classic Masern/Measles Virus IgG assay is recommended for determination of immune status and for detection of intrathecal synthesized IgG antibodies in cerebrospinal fluid for CSF diagnostics. | ||||||||||||||||||
| Research area Infectious Disease | ||||||||||||||||||
| Sample type Serum, plasma, whole blood | ||||||||||||||||||
Components
| ||||||||||||||||||
| Storage 2-8°C | ||||||||||||||||||
| Associated products Measles Antigen (BA102VS) Measles Virus IgG Control Serum (BC102G) Measles Virus IgM ELISA Kit (ESR102M) Measles Virus IgM Control Serum (BC102M) Measles Antigen [premium] (BA102VS-S) | ||||||||||||||||||
| Additional information Additional Serion Kit Information |
| target relevance |
|---|
| Organism Measles morbillivirus (MeV) |
| Protein names Measles Virus |
| Structure and strains The measles virus genome is typically 15,894???nucleotides long and encodes eight proteins. The WHO currently recognises 8 clades of measles (A???H). Subtypes are designed with numerals???A1, D2 etc. Currently, 23 subtypes are recognised. The 450 nucleotides that code for the C???terminal 150 amino acids of N are the minimum amount of sequence data required for genotyping a measles virus isolate. Despite the variety of measles genotypes, there is only one measles serotype. Antibodies to measles bind to the hemagglutinin protein. Thus, antibodies against one genotype (such as the vaccine strain) protect against all other genotypes. |
| Detection and diagnosis Demonstration of IgM antibodies is commonly used as evidence for an acute infection and provides positive test results early on with the onset of symptoms. The demonstration of IgG antibodies serves primarily for immune status and vaccination control as well as for confirmation of Measles Virus infections. The detection of IgG antibodies in CSF samples is particularly recommended in cases of suspected encephalitis and multiple sclerosis. |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| 23114697 | Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life. | Brian A Reikie, Shalena Naidoo, Candice E Ruck, Amy L Slogrove, Corena de Beer, Heleen la Grange, Rozanne C M Adams, Kevin Ho, Kinga Smolen, David P Speert, Mark F Cotton, Wolfgang Preiser, Monika Esser, Tobias R Kollmann | Clin Vaccine Immunol 20:33-8 |
| 23114697 | Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life. | Brian A Reikie, Shalena Naidoo, Candice E Ruck, Amy L Slogrove, Corena de Beer, Heleen la Grange, Rozanne C M Adams, Kevin Ho, Kinga Smolen, David P Speert, Mark F Cotton, Wolfgang Preiser, Monika Esser, Tobias R Kollmann | Clin Vaccine Immunol 20:33-8 |
Protocols
| relevant to this product |
|---|
| ESR102G protocol |
Documents
| # | ||
|---|---|---|
| EO0198 | Safety Data Sheet | QC certificate |
| EP0242 | Safety Data Sheet | QC certificate |
| EQ0056 | Safety Data Sheet | QC certificate |
Only logged in customers who have purchased this product may leave a review.


Reviews
There are no reviews yet.