| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| target | Human Tropical Fever (Chikungunya Virus, Dengue fever virus, Zikavirus) IgM |
| species reactivity | Chikungunya Virus, Dengue fever virus, Zikavirus |
| applications | Lateral flow (dipstick) |
| assay type | Indirect & qualitative |
| available sizes | 20 test kits |
Human Tropical Fever IgM Lateral flow dipstick kit 7873
$487.00
Summary
- Mikrogen diagnostik lateral flow device (dipstick) for research use (RUO)
- Human Tropical Fever IgM Lateral flow dipstick kit 7873
- Suitable for IgG detection
- Ready-to-use
- 20 tests
Human Tropical Fever IgM Lateral flow dipstick kit 7873
| kit | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay type Sandwich assay, lateral flow (dipstick) | ||||||||||||||||||
| Research area Infectious Disease | ||||||||||||||||||
| Sample type Serum, plasma, whole blood | ||||||||||||||||||
Components
| ||||||||||||||||||
| Storage Store at 2-8°C. | ||||||||||||||||||
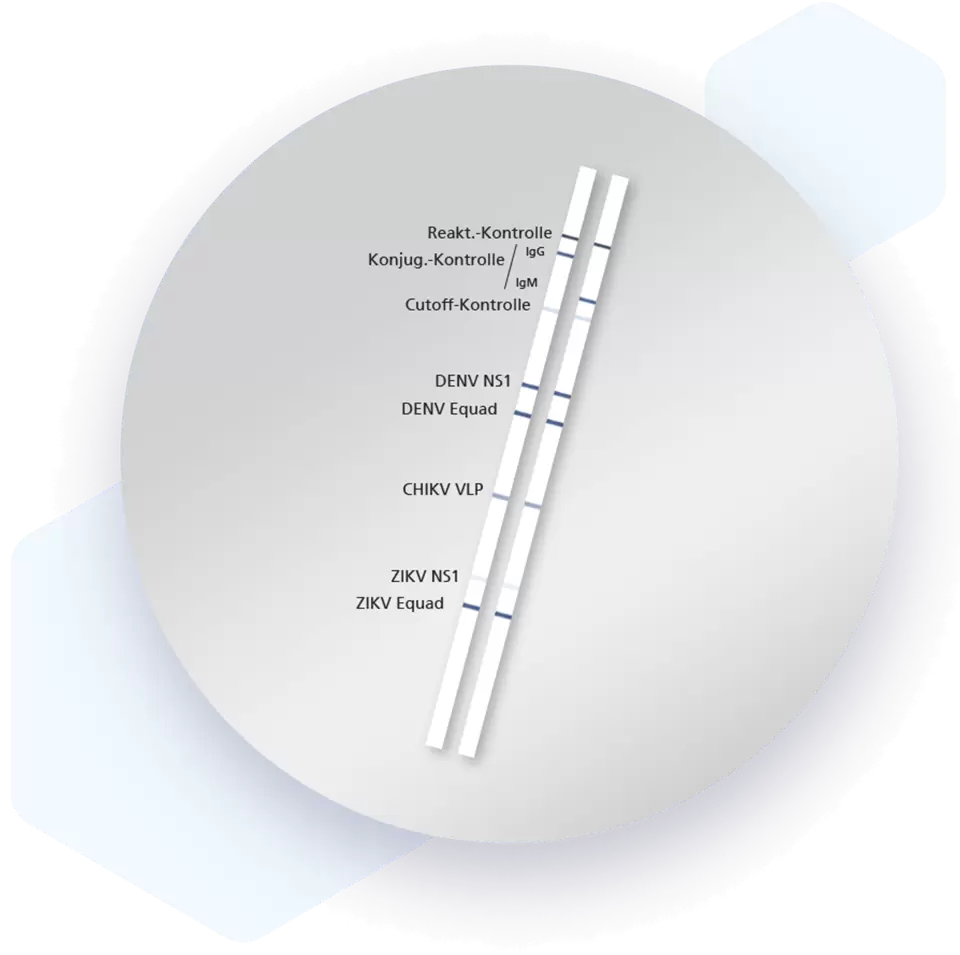
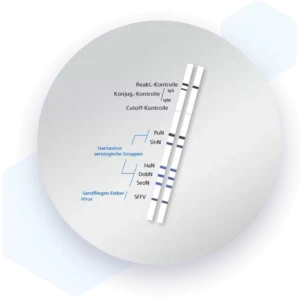
| Additional information Mikrogen recomLine Tropical Fever Test The Mikrogen recomLine Tropical Fever tests are serological, qualitative line immunoassays that combine the diagnostic markers Equad proteins and non-structural proteins (NS1), thereby enabling the identification of specific antibodies against the individual antigens of Dengue, Chikungunya, and Zika viruses. Virus-like particles (VLP) are used to detect CHIKV and NS1 and Equad proteins are used for DENV and ZIKV. The unique setup allows differentiation of DENV and ZIKV using a 2-step interpretation scheme that includes NS1 antigen reactions and, in their absence, reactivities against Equad proteins. Advantages
|
| target relevance |
|---|
| Protein names Troipcal Fever |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| 7873 protocol |
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||
Only logged in customers who have purchased this product may leave a review.






Reviews
There are no reviews yet.