| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| target | Human Parvovirus B19 IgG |
| species reactivity | Human Parvovirus B19 |
| applications | Lateral flow (dipstick) |
| assay type | Indirect & qualitative |
| available sizes | 20 test kits |
Human Parvovirus B19 IgG Lateral flow dipstick kit 4472
$487.00
Summary
- Mikrogen diagnostik lateral flow device (dipstick) for research use (RUO)
- Human Parvovirus B19 IgG Lateral flow dipstick kit 4472
- Suitable for IgG detection
- Ready-to-use
- 20 tests
Human Parvovirus B19 IgG Lateral flow dipstick kit 4472
| kit | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay type Sandwich assay, lateral flow (dipstick) | |||||||||||||||||||||
| Research area Infectious Disease | |||||||||||||||||||||
| Sample type Serum, plasma, whole blood | |||||||||||||||||||||
Components
| |||||||||||||||||||||
| Storage Store at 2-8°C. | |||||||||||||||||||||
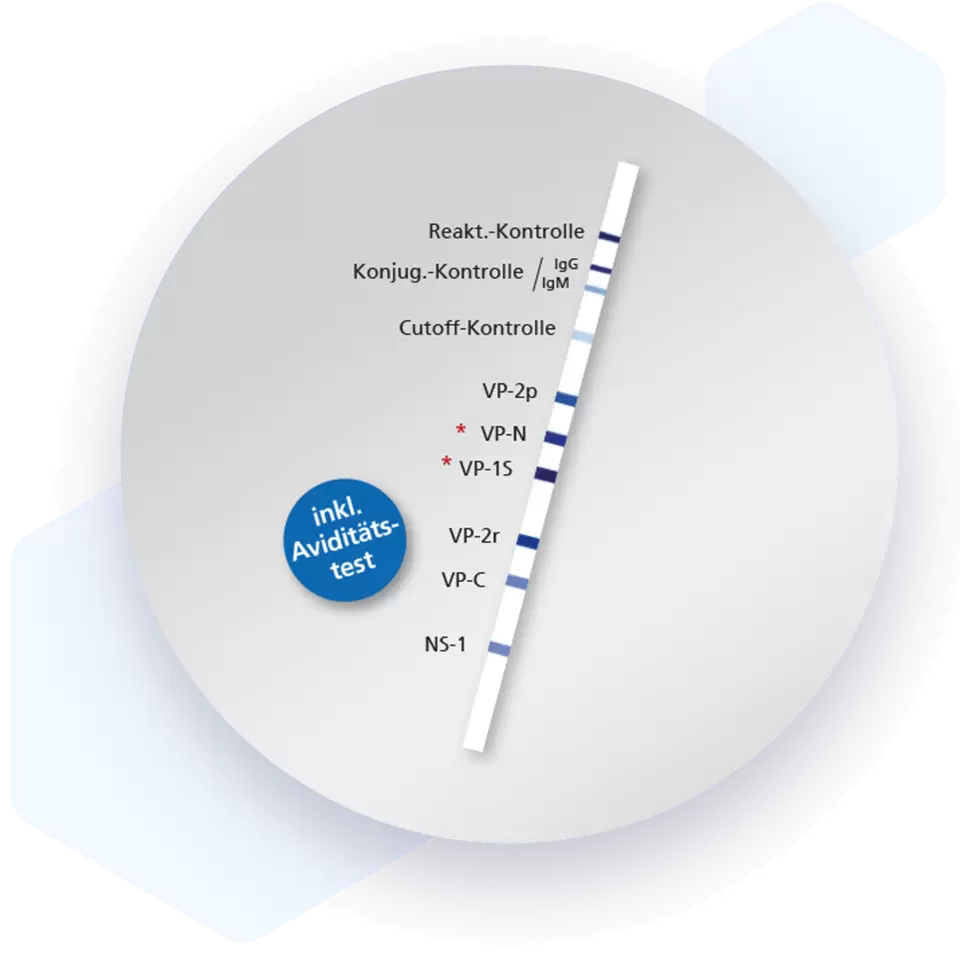
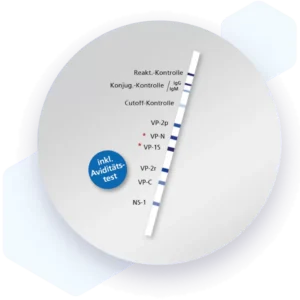
| Additional information Mikrogen recomLine Parvovirus B19 Test The Mikrogen recomLine Parvovirus B19 tests allow an indication of the time of infection due to the reactivity patterns of the recombinant antigens and the possibility to detect avidity determination of the IgG antibodies. The determination of antibodies against the NS-1 antigen can be helpful in clarifying persistent parvovirus B19 infections. By using VP-2 particles, a presentation of conformational epitopes to the otherwise presented linear epitopes is achieved. Advantages
|
| target relevance |
|---|
| Organism Parvovirus B19 |
| Protein names Parvovirus |
| Structure and strains Human parvovirus B19, generally referred to as B19 virus (B19V), parvovirus B19 or sometimes erythrovirus B19, is the first (and until 2005 the only) known human virus in the family Parvoviridae, genus Erythroparvovirus; it measures only 23 26 nm in diameter. Human parvovirus b19 is a below-species classification of Erythroparvovirus primate1. The name is derived from Latin parvum, meaning small, reflecting the fact that B19 ranks among the smallest DNA viruses. B19 virus is most known for causing disease in the pediatric population; however, it can also affect adults. It is the classic cause of the childhood rash called fifth disease or erythema infectiosum, or "slapped cheek syndrome". |
| Detection and diagnosis As a consequence of the variable clinical symptoms which may be associated with Parvovirus B19 infection, a clinical diagnosis should be supported by the demonstration of virus specific antibodies. |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| 4472 protocol |
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||
Only logged in customers who have purchased this product may leave a review.









Reviews
There are no reviews yet.