| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| target | Tropical Fever 1 |
| species reactivity | Detection of Zika virus, chikungunya virus, dengue virus 1-4 and yellow fever virus |
| applications | RT PCR |
| assay type | direct & qualitative |
| available sizes | 96 tests |
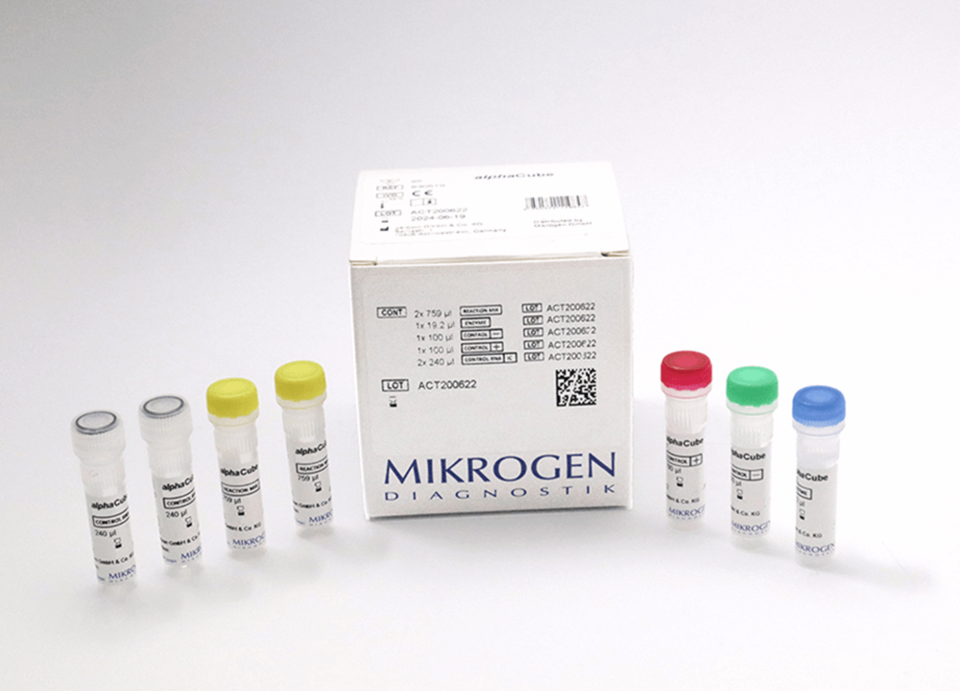
Tropical Fever 1 RT-PCR test Mikrogen 830563
$487.00
Summary
- Mikrogen diagnostik RT PCR kit for research use (RUO)
- Direct Chikungunya Virus, Dengue fever virus, Zikavirus, Yellow fever virus detection
- High sensitivity and specificity
- Internal control for monitoring nucleic acid extraction
(RNA/DNA) and real-time PCR inhibition in each reaction - Compatible with most common real-time PCR cyclers & RNA/DNA extraction methods
- 96 tests
Tropical Fever 1 RT-PCR test Mikrogen 830563
| kit | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay type RT PCR | |||||||||||||||
| Research area Infectious Disease | |||||||||||||||
| Sample type whole blood, serum, plasma, urine, tissue, stool, etc., food and environmental samples or from the carrier material | |||||||||||||||
Notes
| |||||||||||||||
Components
| |||||||||||||||
| Storage Store at -20°C. | |||||||||||||||
| Additional information Highly sensitive and specific direct detection of pathogens that can cause tick-borne infections
Applicable to human starting material as well as RNA/DNA from the tick |
| target relevance |
|---|
| Organism Chikungunya Virus |
| Protein names Chikungunyavirus |
| Structure and strains Chikungunya is an infection caused by the Chikungunya virus (CHIKV). The disease was first identified in 1952 in Tanzania and named based on the Kimakonde words for "to become contorted". Symptoms include fever and joint pain. These typically occur two to twelve days after exposure. Other symptoms may include headache, muscle pain, joint swelling, and a rash. Symptoms usually improve within a week; however, occasionally the joint pain may last for months or years. The risk of death is around 1 in 1,000. The very young, old, and those with other health problems are at risk of more severe disease. |
| Detection and diagnosis The diagnosis is mainly based on direct pathogen detection or serological methods. Within the early phases virus detection by RT-PCR is the method of choice. After the onset of immune responses viremia will drop leading to reduced sensitivity of direct detection methods. Achieving this stage demonstration of specific antibodies should be used for diagnosis. Due to persistence of IgG and to lesser extent IgM antibody serology allows for retrospective evaluation. |
Data
| No results found |
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| 830563 protocol |
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||
Only logged in customers who have purchased this product may leave a review.


Reviews
There are no reviews yet.