| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | NP_034018.2 |
| express system | HEK293 |
| product tag | C-His |
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | Factor H is the major soluble inhibitor of complement, where its binding to self markers (i.e., particular glycan structures) prevents complement activation and amplification on host surfaces. Not surprisingly, mutations and polymorphisms that affect recognition of self by factor H are associated with diseases of complement dysregulation, such as age-related macular degeneration and atypical haemolytic uremic syndrome. In addition, pathogens (i.e., non-self) and cancer cells (i.e., altered-self) can hijack factor H to evade the immune response. |
| molecular weight | The protein has a predicted MW of 42.5 kDa. Due to glycosylation, the protein migrates to 60-65 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per μg by the LAL method. |
Mouse Factor H/CFH Protein 3501
$270.00 – $900.00
Summary
- Expression: HEK293
- Pure: Yes (HPLC)
- Amino Acid Range: Ser875-Val1252
Mouse Factor H/CFH Protein 3501
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Factor H is the major soluble inhibitor of complement, where its binding to self markers (i.e., particular glycan structures) prevents complement activation and amplification on host surfaces. Not surprisingly, mutations and polymorphisms that affect recognition of self by factor H are associated with diseases of complement dysregulation, such as age-related macular degeneration and atypical haemolytic uremic syndrome. In addition, pathogens (i.e., non-self) and cancer cells (i.e., altered-self) can hijack factor H to evade the immune response. |
| Protein names Complement factor H (H factor 1) |
| Mass 139096Da |
| Function Glycoprotein that plays an essential role in maintaining a well-balanced immune response by modulating complement activation. Acts as a soluble inhibitor of complement, where its binding to self markers such as glycan structures prevents complement activation and amplification on cell surfaces (PubMed:21285368, PubMed:25402769). Accelerates the decay of the complement alternative pathway (AP) C3 convertase C3bBb, thus preventing local formation of more C3b, the central player of the complement amplification loop (PubMed:19503104, PubMed:26700768). As a cofactor of the serine protease factor I, CFH also regulates proteolytic degradation of already-deposited C3b (PubMed:18252712, PubMed:23332154, PubMed:28671664). In addition, mediates several cellular responses through interaction with specific receptors. For example, interacts with CR3/ITGAM receptor and thereby mediates the adhesion of human neutrophils to different pathogens. In turn, these pathogens are phagocytosed and destroyed (PubMed:20008295, PubMed:9558116). {ECO:0000269|PubMed:18252712, ECO:0000269|PubMed:19503104, ECO:0000269|PubMed:20008295, ECO:0000269|PubMed:21285368, ECO:0000269|PubMed:23332154, ECO:0000269|PubMed:25402769, ECO:0000269|PubMed:26700768, ECO:0000269|PubMed:28671664, ECO:0000269|PubMed:9558116}.; (Microbial infection) In the mosquito midgut, binds to the surface of parasite P.falciparum gametocytes and protects the parasite from alternative complement pathway-mediated elimination. {ECO:0000269|PubMed:23332154}. |
| Subellular location Secreted.; Note=(Microbial infection) In the mosquito midgut, localizes to P.falciparum (NF54 strain) macrogamete and young zygote cell membranes. {ECO:0000269|PubMed:23332154}. |
| Tissues Expressed in the retinal pigment epithelium (at protein level) (PubMed:25136834). CFH is one of the most abundant complement components in blood where the liver is the major source of CFH protein in vivo. in addition, CFH is secreted by additional cell types including monocytes, fibroblasts, or endothelial cells (PubMed:2139673, PubMed:25136834, PubMed:2968404, PubMed:6444659). {ECO:0000269|PubMed:2139673, ECO:0000269|PubMed:25136834, ECO:0000269|PubMed:2968404, ECO:0000269|PubMed:6444659}. |
| Structure Homodimer (PubMed:18005991, PubMed:19505476). Forms also homooligomers (PubMed:19505476). Interacts with complement protein C3b; this interaction inhibits complement activation (PubMed:16601698, PubMed:19503104, PubMed:20378178, PubMed:21285368, PubMed:28671664). Interacts with complement protein C3d (PubMed:20378178, PubMed:21285368, PubMed:29190743). Interacts with CR3/ITGAM; this interaction mediates adhesion of neutrophils to pathogens leading to pathogen clearance (PubMed:20008295, PubMed:9558116). Interacts with complement factor I (PubMed:28671664). {ECO:0000269|PubMed:16601698, ECO:0000269|PubMed:18005991, ECO:0000269|PubMed:19503104, ECO:0000269|PubMed:19505476, ECO:0000269|PubMed:20008295, ECO:0000269|PubMed:20378178, ECO:0000269|PubMed:21285368, ECO:0000269|PubMed:28671664, ECO:0000269|PubMed:29190743, ECO:0000269|PubMed:9558116}.; (Microbial infection) Interacts with West nile virus non-structural protein 1 (NS1); this interaction leads to the degradation of C3. {ECO:0000269|PubMed:17132743}.; (Microbial infection) Interacts with C.albicans GPD2; the interaction is direct and leads to the degradation of C3 which enables the pathogen to evade the host innate immune system. {ECO:0000269|PubMed:23204165, ECO:0000269|PubMed:34986357}.; (Microbial infection) Interacts with Neisseria meningitidis protein fHbp. {ECO:0000269|PubMed:19225461, ECO:0000269|PubMed:23133374}.; (Microbial infection) Interacts with Borrelia burgdorferi outer surface protein E/OspE; this interaction recruits complement regulator factor H onto the bacterial surface to evade complement-mediated cell lysis. {ECO:0000269|PubMed:23658013, ECO:0000269|PubMed:29190743}.; (Microbial infection) Interacts with Streptococcus pneumoniae protein virulence factor choline-binding protein A/CbpAN; this interaction enables Streptococcus pneumoniae to evade surveillance by human complement system. {ECO:0000269|PubMed:25330773}.; (Microbial infection) Interacts with Staphylococcus aureus surface protein serine-aspartate repeat protein E/SdrE; this interaction sequesters CFH on the surface of S.aureus for complement evasion. {ECO:0000269|PubMed:28258151}.; (Microbial infection) Interacts with Staphylococcus aureus protein Sbi; this interaction inhibits the complement activation of the alternative pathway. {ECO:0000269|PubMed:19112495}.; [Isoform 1]: (Microbial infection) Interacts (via sushi 4-6 domains) with P.falciparum surface protein PF92; the interaction recruits CFH onto the merozoite surface preventing complement-mediated cell lysis (PubMed:26700768). The interaction does not affect CFH activity (PubMed:26700768). Interacts (via sushi 6-7 domains) with P.falciparum (strain NF54) GAP50; the interaction occurs in the vector mosquito midgut at the surface of the activated parasite gametocytes; the interaction protects the parasite from alternative complement pathway-mediated elimination (PubMed:23332154). {ECO:0000269|PubMed:23332154, ECO:0000269|PubMed:26700768}.; [Isoform 2]: (Microbial infection) Interacts (via sushi 4-6 domains) with P.falciparum surface protein PF92; the interaction recruits FHL-1 isoform onto the merozoite surface preventing complement-mediated cell lysis (PubMed:26700768). The interaction does not affect FHL-1 isoform activity (PubMed:26700768). Interacts (via sushi 6-7 domains) with P.falciparum (strain NF54) GAP50; the interaction occurs in the vector mosquito midgut at the surface of the activated parasite gametocytes; the interaction protects the parasite from alternative complement pathway-mediated elimination (PubMed:23332154). {ECO:0000269|PubMed:23332154, ECO:0000269|PubMed:26700768}. |
| Post-translational modification Sulfated on tyrosine residues. {ECO:0000269|PubMed:25136834}.; According to a report, Asn-217 is not glycosylated (PubMed:17591618). Another study observed glycosylation at this position (PubMed:19139490). {ECO:0000305|PubMed:17591618, ECO:0000305|PubMed:19139490}. |
| Domain Sushi 1-3 domain represents the minimal unit capable of cofactor activity (PubMed:18252712). |
| Target Relevance information above includes information from UniProt accession: P08603 |
| The UniProt Consortium |
Data
 |
| The purity of Mouse Factor H/CFH is greater than 95% as determined by SEC-HPLC. |
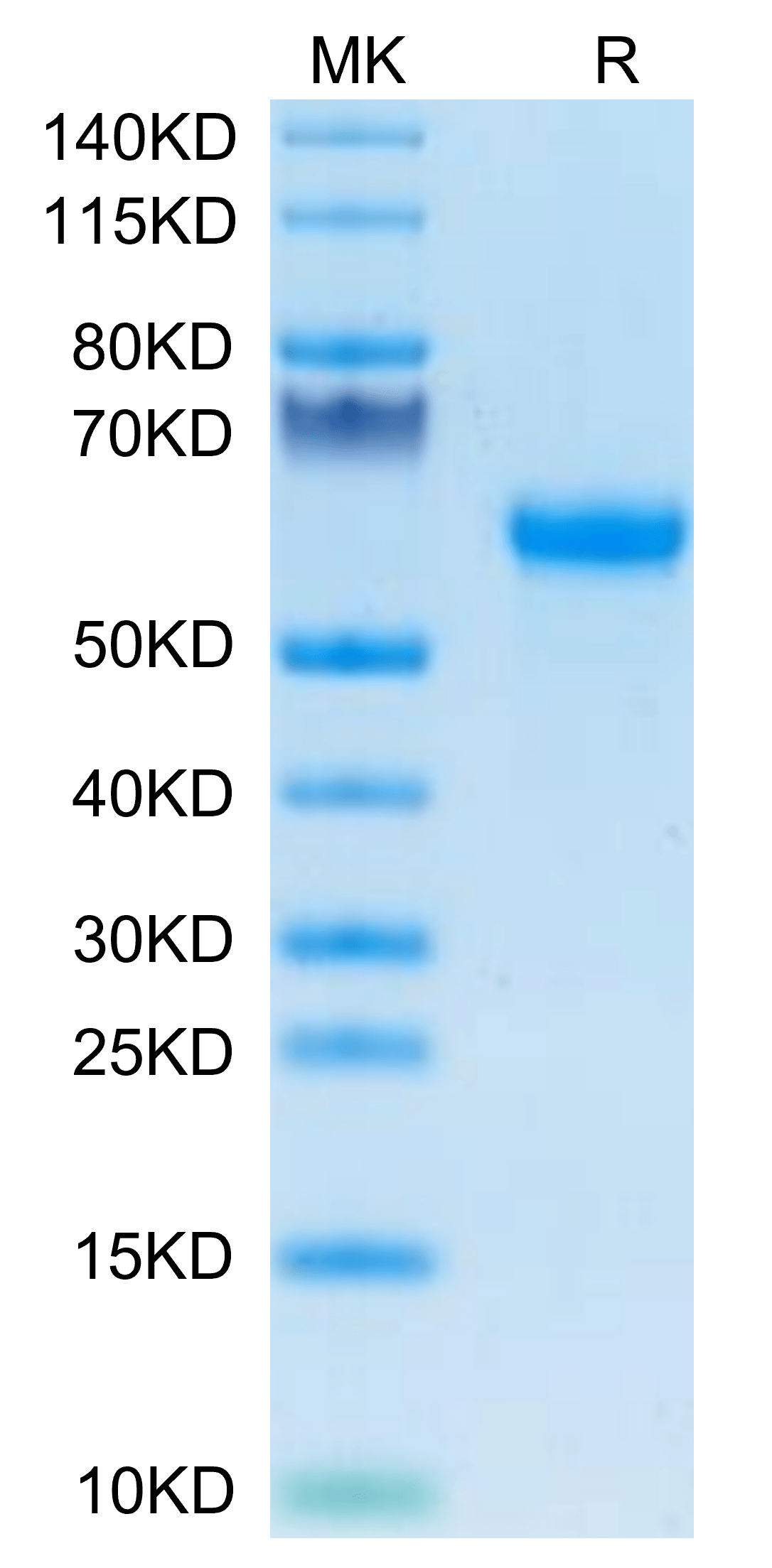 |
| Mouse Factor H/CFH on Tris-Bis PAGE under reduced condition. The purity is greater than 95%. |
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||














