| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| target | Human SARS-CoV-2 IgG |
| species reactivity | Human SARS-CoV-2 |
| applications | Lateral flow (dipstick) |
| assay type | Indirect & qualitative |
| available sizes | 20 test kits |
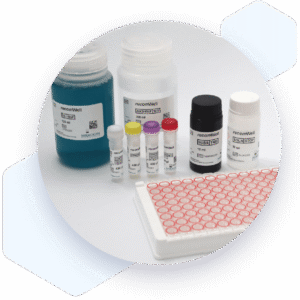
Human SARS-CoV-2 IgG Lateral flow dipstick kit 7374
$487.00
Summary
- Mikrogen diagnostik lateral flow device (dipstick) for research use (RUO)
- Human SARS-CoV-2 IgG Lateral flow dipstick kit 7374
- Suitable for IgG detection
- Ready-to-use
- 20 tests
Human SARS-CoV-2 IgG Lateral flow dipstick kit 7374
| kit | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay type Sandwich assay, lateral flow (dipstick) | ||||||||||||||||||
| Research area Infectious Disease | ||||||||||||||||||
| Sample type Serum, plasma, whole blood | ||||||||||||||||||
Components
| ||||||||||||||||||
| Storage Store at 2-8°C. | ||||||||||||||||||
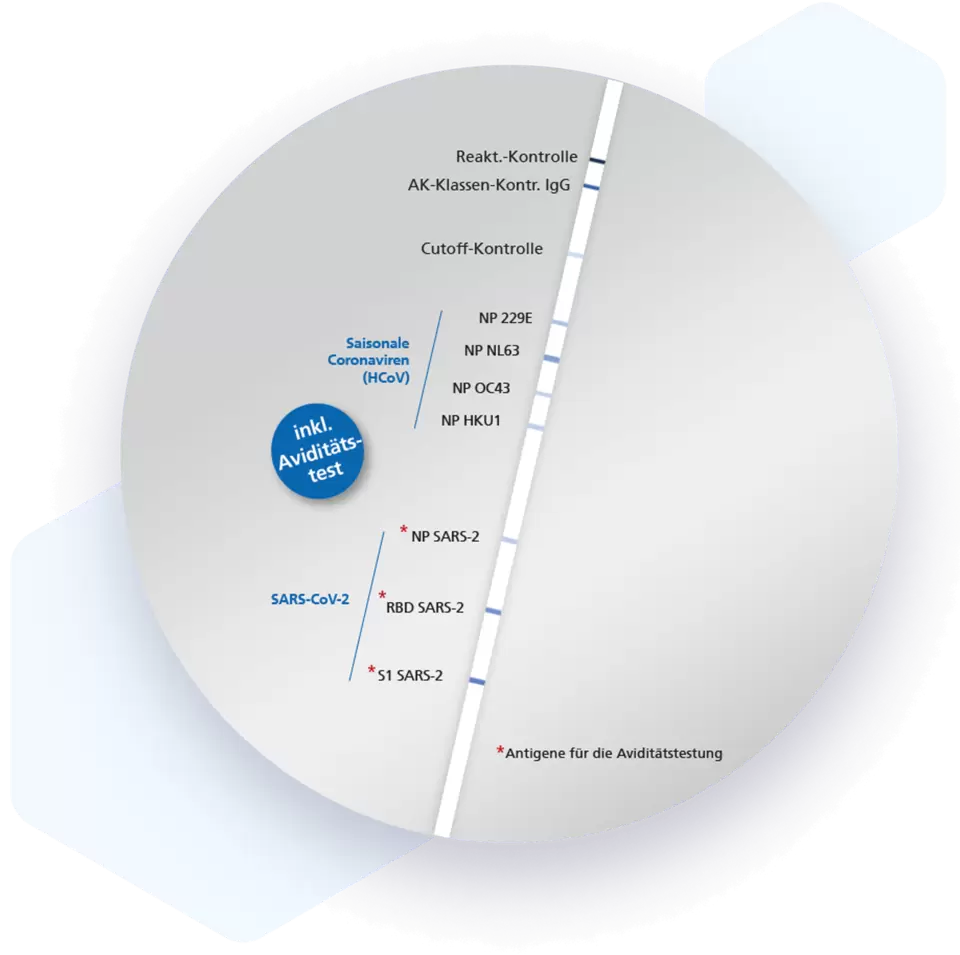
| Additional information Mikrogen recomLine SARS-CoV-2 IgG [Avidity] Test In commercial screening tests for antibody detection, the nucleocapsid protein (NP) and/or the spike protein (S) or corresponding subunits are usually used as immunodominant antigens. The Mikrogen recomLine SARS-CoV-2 IgG [avidity] line immunoassay combines these diagnostic markers and allows the identification of specific antibodies against the individual antigens. In addition, reactivities against the seasonal coronaviruses (HCoV) are detected. The test allows the assessment of the adaptive humoral immune response to SARS-CoV-2 after infection and/or vaccination. An added benefit is that this test system determines the quality of the antibodies when avidity measurement is used. Advantages
|
| target relevance |
|---|
| Organism SARS-CoV-2 |
| Protein names SARS-CoV-2 |
| Structure and strains SARS-CoV-2 consists of 4 structural proteins and 16 non-structural proteins. The 4 structural proteins are spike (S), envelope (E), nucleocapsid (N) and membrane (M) protein. The N protein is associated with the RNA genome, the S, E and M proteins together form the virus envelope. The S protein is a glycoprotein consisting of two domains, the S1 domain, which contains the receptor binding domain (RBD) responsible for the virus being able to attach to and fuse with the ACE2 receptor of the host cell, and the S2 domain including transmembrane and endodomain. On the virus surface, the S protein forms trimeric structures. N and S protein are major immunogenic proteins of the virus family Coronaviridae and are very well suited for serological detection of anti-SARS-CoV-2 antibodies. Recombinant versions of both proteins are already widely used in different test systems. |
| Detection and diagnosis Neutralizing antibodies during host immune responses are predominantly directed against S protein, which is supposedly suitable for very specific test settings as it is less conserved within the family of Coronaviridae than the other immunogenic antigens. However, recent studies have shown that antibodies against N protein are detectable earlier in mild infections than antibodies directed against S protein. As an acute marker in particular, it can be beneficial to use N protein for most sensitive early detection. In the further course of the immune response, the N protein antibody level decreases, whereas antibodies against S protein are detectable for a longer time. Various studies show that anti-SARS-CoV-2 antibodies of the IgA and IgM class are detectable in the median at the earliest from about six days after the onset of symptoms, increase in the further course of the disease and decrease again between 18 and 35 days. Antibodies of the IgG class are detectable in the median ten to eighteen days after onset of symptoms and are detectable over several months. |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| #N/A |
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||
Only logged in customers who have purchased this product may leave a review.








Reviews
There are no reviews yet.