| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | NP_001531 |
| express system | E.coli |
| product tag | C-His |
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | Heat shock protein beta-1 (HSPB1, also known as HSP27) is a small heat shock protein involved in many cellular processes and reportedly protects cells against oxidative stress. This protein is expressed only in insulin-dependent tissues (heart, skeletal muscle, and fat tissue), and expression of HspB7 is regulated by many different factors. HspB7 has an unusual N-terminal sequence, a conservative ╬▒-crystallin domain, and very short C-terminal domain lacking conservative IPV tripeptide involved in a small heat shock proteins oligomer formation. |
| molecular weight | The protein has a predicted MW of 23.74 kDa. The protein migrates to 25-30 kDa based on Tris-Bis PAGE result. |
| available size | 100 ┬Ág, 500 ┬Ág |
| endotoxin | Less than 1EU per ╬╝g by the LAL method. |
Human Hsp27/HSPB1 Protein 2459
$300.00 – $1,000.00
Summary
- Expression: E.coli
- Pure: Yes (HPLC)
- Amino Acid Range: Met1-Lys205
Human Hsp27/HSPB1 Protein 2459
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Heat shock protein beta-1 (HSPB1, also known as HSP27) is a small heat shock protein involved in many cellular processes and reportedly protects cells against oxidative stress. This protein is expressed only in insulin-dependent tissues (heart, skeletal muscle, and fat tissue), and expression of HspB7 is regulated by many different factors. HspB7 has an unusual N-terminal sequence, a conservative α-crystallin domain, and very short C-terminal domain lacking conservative IPV tripeptide involved in a small heat shock proteins oligomer formation. |
| Protein names Heat shock protein beta-1 (HspB1) (28 kDa heat shock protein) (Estrogen-regulated 24 kDa protein) (Heat shock 27 kDa protein) (HSP 27) (Heat shock protein family B member 1) (Stress-responsive protein 27) (SRP27) |
| Protein family Small heat shock protein (HSP20) family |
| Mass 22783Da |
| Function Small heat shock protein which functions as a molecular chaperone probably maintaining denatured proteins in a folding-competent state (PubMed:10383393, PubMed:20178975). Plays a role in stress resistance and actin organization (PubMed:19166925). Through its molecular chaperone activity may regulate numerous biological processes including the phosphorylation and the axonal transport of neurofilament proteins (PubMed:23728742). {ECO:0000269|PubMed:10383393, ECO:0000269|PubMed:19166925, ECO:0000269|PubMed:20178975, ECO:0000269|PubMed:23728742}. |
| Subellular location Cytoplasm {ECO:0000269|PubMed:10777697, ECO:0000269|PubMed:28144995}. Nucleus {ECO:0000269|PubMed:19464326}. Cytoplasm, cytoskeleton, spindle {ECO:0000269|PubMed:10777697}. Note=Cytoplasmic in interphase cells. Colocalizes with mitotic spindles in mitotic cells. Translocates to the nucleus during heat shock and resides in sub-nuclear structures known as SC35 speckles or nuclear splicing speckles. {ECO:0000269|PubMed:19464326}. |
| Tissues Detected in all tissues tested: skeletal muscle, heart, aorta, large intestine, small intestine, stomach, esophagus, bladder, adrenal gland, thyroid, pancreas, testis, adipose tissue, kidney, liver, spleen, cerebral cortex, blood serum and cerebrospinal fluid. Highest levels are found in the heart and in tissues composed of striated and smooth muscle. {ECO:0000269|PubMed:1560006}. |
| Structure Homooligomer (PubMed:10383393). Homodimer; becomes monomeric upon activation (PubMed:20178975). Heterooligomer; with HSPB6 (PubMed:23948568). Associates with alpha- and beta-tubulin (PubMed:10777697). Interacts with TGFB1I1 (By similarity). Interacts with CRYAB (PubMed:1560006). Interacts with HSPB8 (PubMed:11342557). Interacts with HSPBAP1 (PubMed:10751411). {ECO:0000250|UniProtKB:P42930, ECO:0000269|PubMed:10383393, ECO:0000269|PubMed:10751411, ECO:0000269|PubMed:10777697, ECO:0000269|PubMed:11342557, ECO:0000269|PubMed:1560006, ECO:0000269|PubMed:20178975, ECO:0000269|PubMed:23948568}. |
| Post-translational modification Phosphorylated upon exposure to protein kinase C activators and heat shock (PubMed:8325890). Phosphorylation by MAPKAPK2 and MAPKAPK3 in response to stress dissociates HSPB1 from large small heat-shock protein (sHsps) oligomers and impairs its chaperone activity and ability to protect against oxidative stress effectively. Phosphorylation by MAPKAPK5 in response to PKA stimulation induces F-actin rearrangement (PubMed:1332886, PubMed:19166925, PubMed:8093612). {ECO:0000269|PubMed:1332886, ECO:0000269|PubMed:19166925, ECO:0000269|PubMed:8093612, ECO:0000269|PubMed:8325890}. |
| Target Relevance information above includes information from UniProt accession: P04792 |
| The UniProt Consortium |
Data
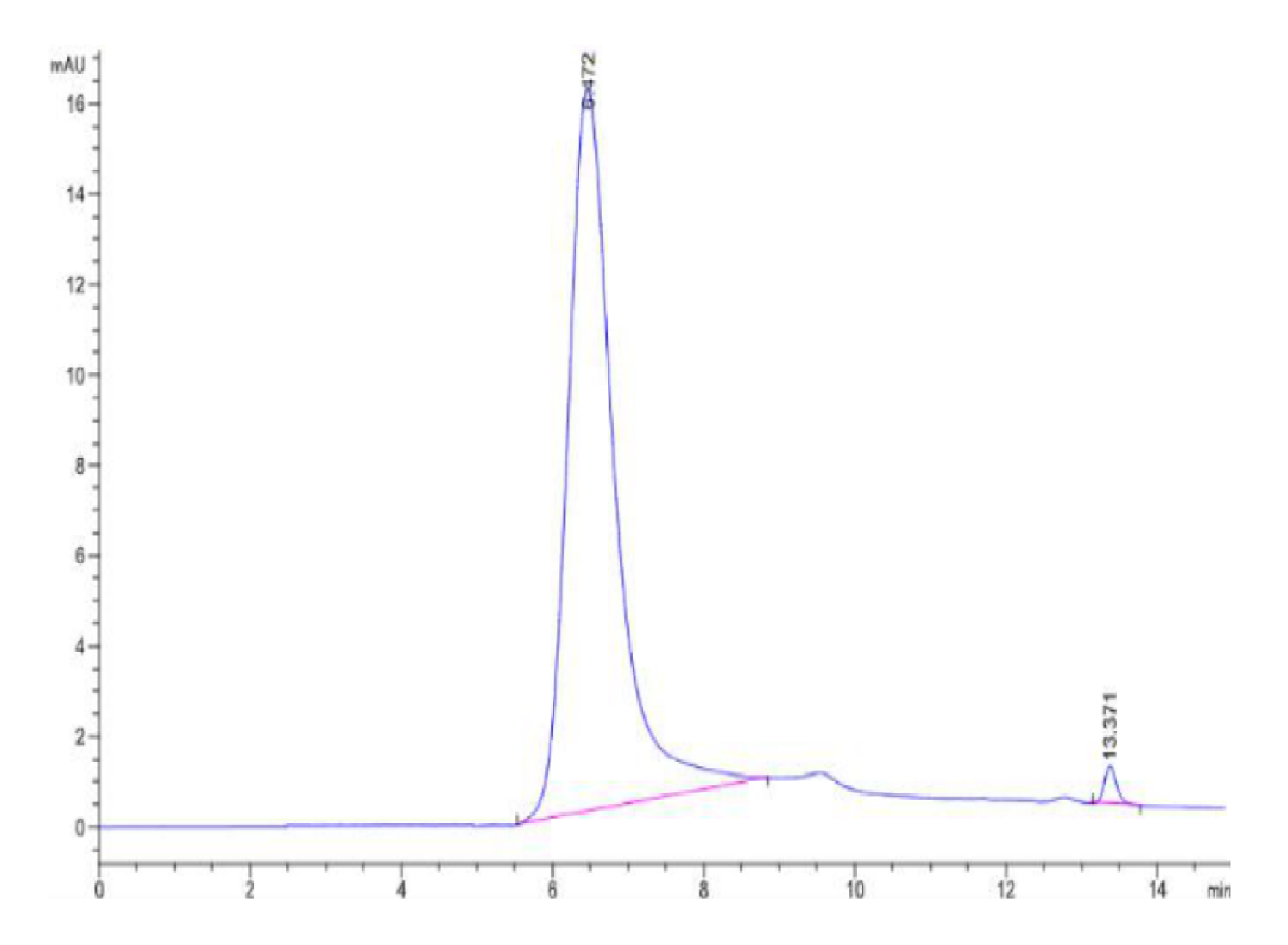 |
| The purity of Human Hsp27 is greater than 95% as determined by SEC-HPLC. |
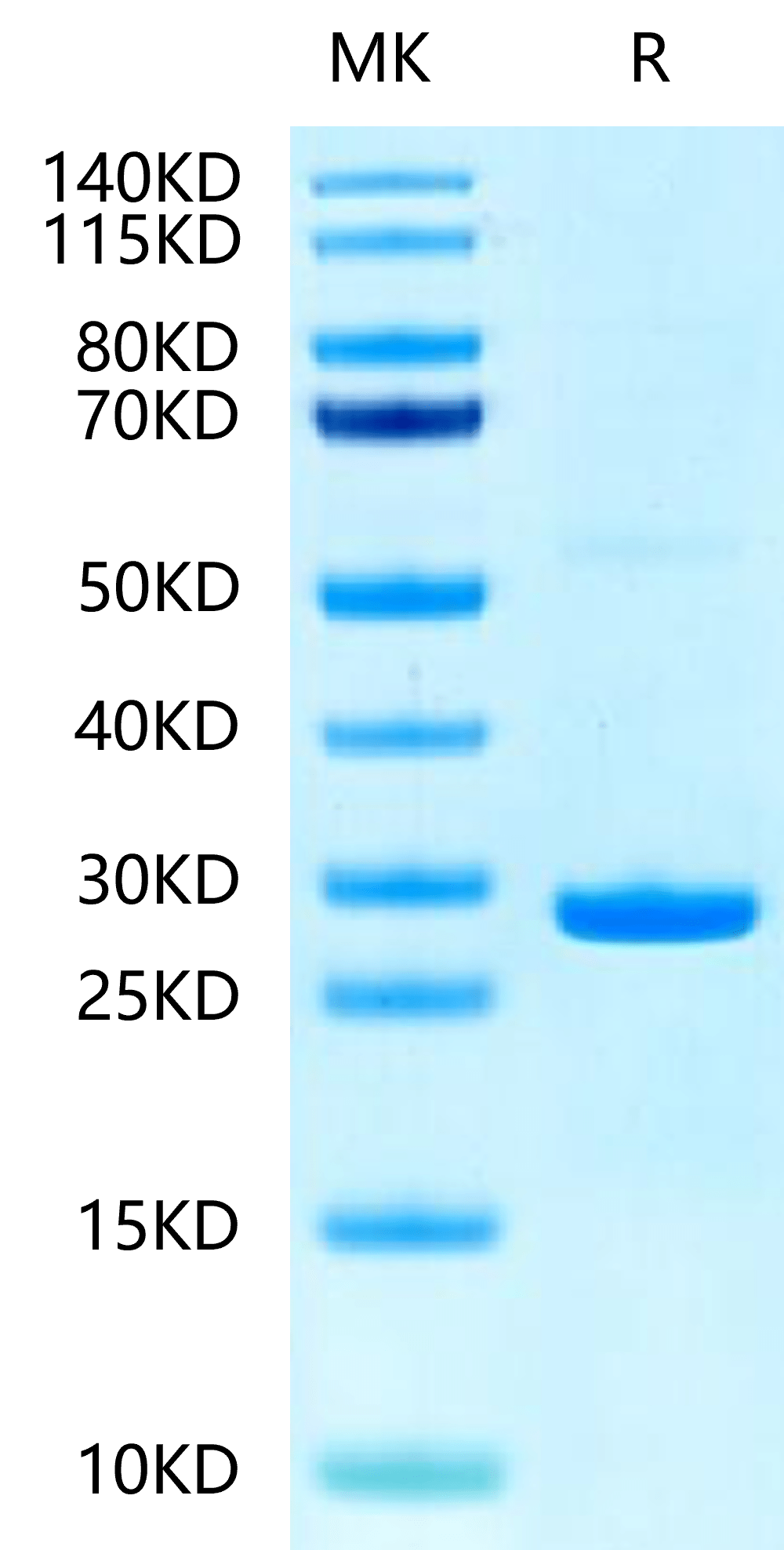 |
| Human Hsp27 on Tris-Bis PAGE under reduced condition. The purity is greater than 95%. |
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||














