| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | Q13241 |
| express system | HEK293 |
| product tag | N-His-Avi |
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | CD94 is an approximately 25 kDa type 2 transmembrane protein that plays an important role in regulating natural killer (NK) cell activation.CD94 plays a role as a receptor for the recognition of MHC class I HLA-E molecules by NK cells and some cytotoxic T-cells. |
| molecular weight | The protein has a predicted MW of 19.8 kDa. Due to glycosylation, the protein migrates to 38-42 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per μg by the LAL method. |
Human CD94 Protein 4535
$345.00 – $1,150.00
Summary
- Expression: HEK293
- Pure: Yes (HPLC)
- Amino Acid Range: Ser34-Ile179
Human CD94 Protein 4535
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| CD94 is an approximately 25 kDa type 2 transmembrane protein that plays an important role in regulating natural killer (NK) cell activation.CD94 plays a role as a receptor for the recognition of MHC class I HLA-E molecules by NK cells and some cytotoxic T-cells. |
| Protein names Natural killer cells antigen CD94 (KP43) (Killer cell lectin-like receptor subfamily D member 1) (NK cell receptor) (CD antigen CD94) |
| Gene names KLRD1,KLRD1 CD94 |
| Mass 9606Da |
| Function Immune receptor involved in self-nonself discrimination. In complex with KLRC1 or KLRC2 on cytotoxic and regulatory lymphocyte subsets, recognizes non-classical major histocompatibility (MHC) class Ib molecule HLA-E loaded with self-peptides derived from the signal sequence of classical MHC class Ia and non-classical MHC class Ib molecules (PubMed:10023772, PubMed:18064301, PubMed:18083576, PubMed:37264229, PubMed:9486650, PubMed:9754572). Enables cytotoxic cells to monitor the expression of MHC class I molecules in healthy cells and to tolerate self (PubMed:12387742, PubMed:18064301, PubMed:9430220). Primarily functions as a ligand binding subunit as it lacks the capacity to signal.; KLRD1-KLRC1 acts as an immune inhibitory receptor. Key inhibitory receptor on natural killer (NK) cells that regulates their activation and effector functions (PubMed:30860984, PubMed:9430220, PubMed:9485206, PubMed:9486650). Dominantly counteracts T cell receptor signaling on a subset of memory/effector CD8-positive T cells as part of an antigen-driven response to avoid autoimmunity (PubMed:12387742). On intraepithelial CD8-positive gamma-delta regulatory T cells triggers TGFB1 secretion, which in turn limits the cytotoxic programming of intraepithelial CD8-positive alpha-beta T cells, distinguishing harmless from pathogenic antigens (PubMed:18064301). In HLA-E-rich tumor microenvironment, acts as an immune inhibitory checkpoint and may contribute to progressive loss of effector functions of NK cells and tumor-specific T cells, a state known as cell exhaustion (PubMed:30503213, PubMed:30860984). Upon HLA-E-peptide binding, transmits intracellular signals through KLRC1 immunoreceptor tyrosine-based inhibition motifs (ITIMs) by recruiting INPP5D/SHIP-1 and INPPL1/SHIP-2 tyrosine phosphatases to ITIMs, and ultimately opposing signals transmitted by activating receptors through dephosphorylation of proximal signaling molecules (PubMed:12165520, PubMed:9485206).; KLRD1-KLRC2 acts as an immune activating receptor (PubMed:15940674, PubMed:9655483). On cytotoxic lymphocyte subsets recognizes HLA-E loaded with signal sequence-derived peptides from non-classical MHC class Ib HLA-G molecules, likely playing a role in the generation and effector functions of adaptive NK cells and in maternal-fetal tolerance during pregnancy (PubMed:30134159, PubMed:9754572). Regulates the effector functions of terminally differentiated cytotoxic lymphocyte subsets, and in particular may play a role in adaptive NK cell response to viral infection (PubMed:20952657, PubMed:21825173). Upon HLA-E-peptide binding, transmits intracellular signals via the adapter protein TYROBP/DAP12, triggering the phosphorylation of proximal signaling molecules and cell activation (PubMed:15940674, PubMed:9655483).; (Microbial infection) Viruses like human cytomegalovirus have evolved an escape mechanism whereby virus-induced down-regulation of host MHC class I molecules is coupled to the binding of viral peptides to HLA-E, restoring HLA-E expression and inducing HLA-E-dependent NK cell immune tolerance to infected cells. Recognizes HLA-E in complex with human cytomegalovirus UL40-derived peptide (VMAPRTLIL) and inhibits NK cell cytotoxicity.; (Microbial infection) May recognize HLA-E in complex with HIV-1 gag/Capsid protein p24-derived peptide (AISPRTLNA) on infected cells and may inhibit NK cell cytotoxicity, a mechanism that allows HIV-1 to escape immune recognition.; (Microbial infection) Upon SARS-CoV-2 infection, may contribute to functional exhaustion of cytotoxic NK cells and CD8-positive T cells (PubMed:32859121). On NK cells, may recognize HLA-E in complex with SARS-CoV-2 S/Spike protein S1-derived peptide (LQPRTFLL) expressed on the surface of lung epithelial cells, inducing NK cell exhaustion and dampening antiviral immune surveillance (PubMed:32859121). |
| Catalytic activity #N/A |
| Subellular location Cell membrane ; Single-pass type II membrane protein . |
| Tissues Expressed in NK cell subsets (at protein level) (PubMed:21825173, PubMed:9430220, PubMed:9485206). Expressed in memory/effector CD8-positive alpha-beta T cell subsets (at protein level) (PubMed:12387742, PubMed:20952657). Expressed in melanoma-specific cytotoxic T cell clones (at protein level) (PubMed:9485206). Expressed in terminally differentiated cytotoxic gamma-delta T cells (at protein level) (PubMed:20952657). KLRD1-KLRC1 and KLRD1-KLRC2 are differentially expressed in NK and T cell populations, with only minor subsets expressing both receptor complexes (at protein level) (PubMed:20952657). |
| Structure Can form disulfide-bonded heterodimer with NKG2 family members KLRC1 and KLRC2 (PubMed:18083576, PubMed:18332182, PubMed:18448674, PubMed:9655483). KLRD1-KLRC1 heterodimer interacts with peptide-bound HLA-E-B2M heterotrimeric complex. KLRD1 plays a prominent role in directly interacting with HLA-E (PubMed:18083576). KLRD1-KLRC1 interacts with much higher affinity with peptide-bound HLA-E-B2M than KLRD1-KLRC2 (PubMed:10428963, PubMed:9486650). Interacts with the adapter protein TYROBP/DAP12; this interaction is required for cell surface expression and cell activation (PubMed:15940674, PubMed:9655483). |
| Target Relevance information above includes information from UniProt accession: Q13241 |
| The UniProt Consortium |
Data
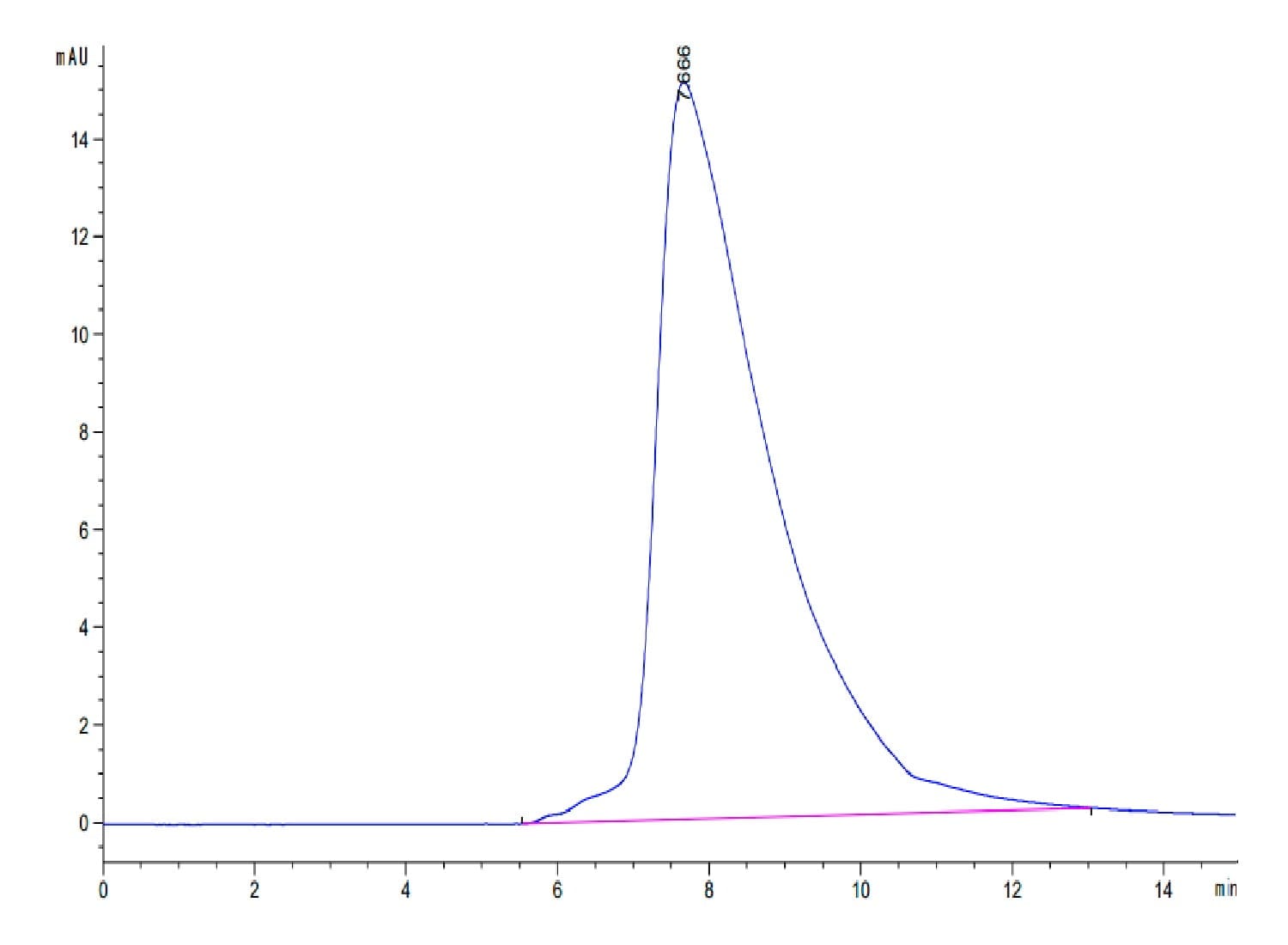 |
| The purity of Human CD94 is greater than 95% as determined by SEC-HPLC. |
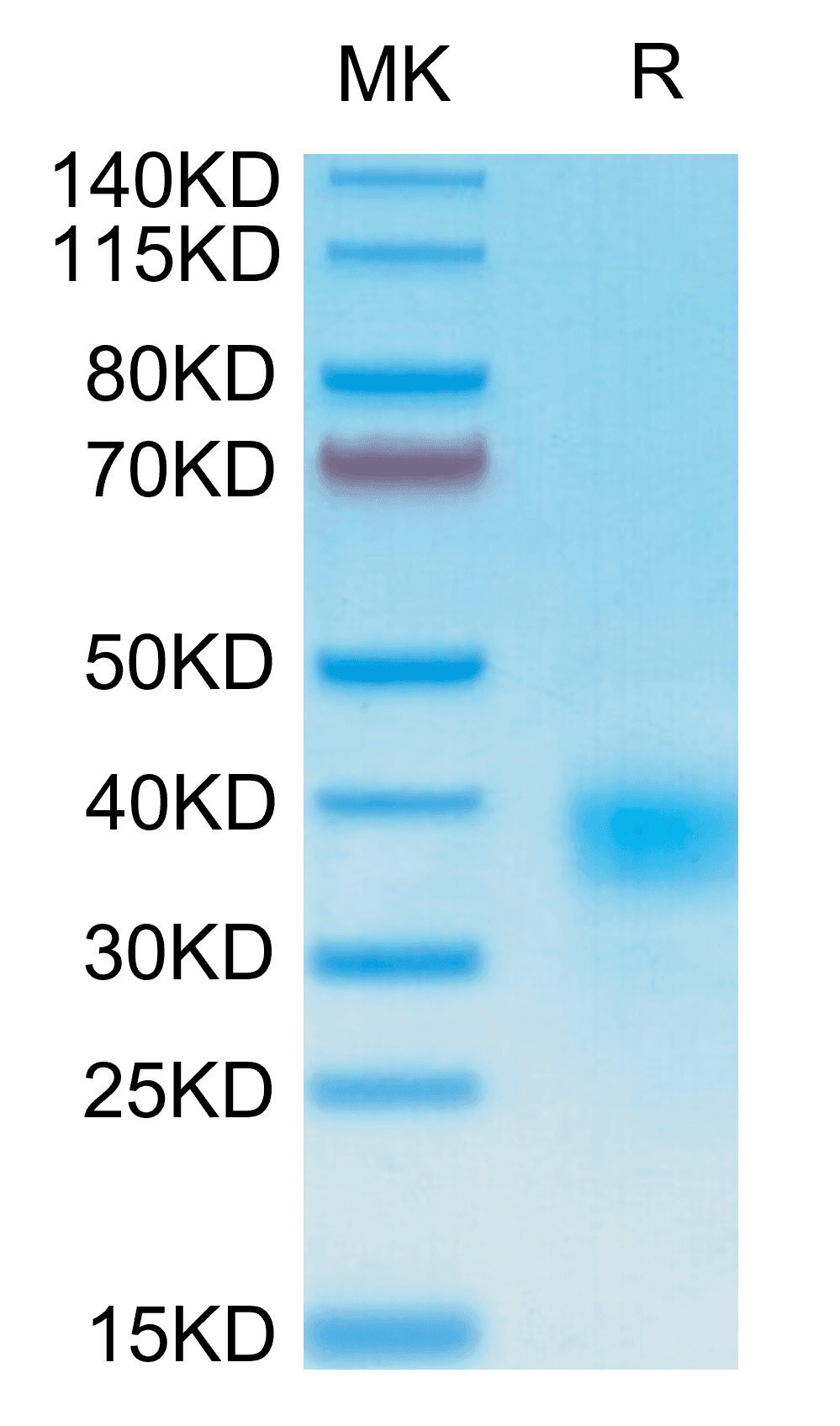 |
| Human CD94 on Tris-Bis PAGE under reduced condition. The purity is greater than 95%. |
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||














