| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | P30566 |
| express system | E.coli |
| product tag | N-His |
| purity | > 95% as determined by Tris-Bis PAGE |
| background | Adenylosuccinate lyase (ADSL) is an essential enzyme for de novo purine biosynthesis, ADSL functions in de novo purine synthesis (DNPS) and the purine nucleotide cycle. Adenylosuccinate lyase ADSL) deficiency is a defect of purine metabolism affecting purinosome assembly and reducing metabolite fluxes through purine de novo synthesis and purine nucleotide recycling pathways. |
| molecular weight | The protein has a predicted MW of 56.28 kDa same as Tris-Bis PAGE result. |
| available size | 100 ¬Ķg, 500 ¬Ķg |
| endotoxin | Less than 1EU per őľg by the LAL method. |
Human Adenylosuccinate Lyase Protein 2466
$300.00 – $1,000.00
Summary
- Expression: E.coli
- Pure: Yes (SDS-PAGE)
- Amino Acid Range: Ala2-Leu484
Human Adenylosuccinate Lyase Protein 2466
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Adenylosuccinate lyase (ADSL) is an essential enzyme for de novo purine biosynthesis, ADSL functions in de novo purine synthesis (DNPS) and the purine nucleotide cycle. Adenylosuccinate lyase ADSL) deficiency is a defect of purine metabolism affecting purinosome assembly and reducing metabolite fluxes through purine de novo synthesis and purine nucleotide recycling pathways. |
| Protein names Adenylosuccinate lyase (ADSL) (ASL) (EC 4.3.2.2) (Adenylosuccinase) (ASase) |
| Gene names ADSL,ADSL AMPS |
| Protein family Lyase 1 family, Adenylosuccinate lyase subfamily |
| Mass 9606Da |
| Function Catalyzes two non-sequential steps in de novo AMP synthesis: converts (S)-2-(5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamido)succinate (SAICAR) to fumarate plus 5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide, and thereby also contributes to de novo IMP synthesis, and converts succinyladenosine monophosphate (SAMP) to AMP and fumarate. |
| Catalytic activity #N/A |
| Pathway PATHWAY: Purine metabolism; AMP biosynthesis via de novo pathway; AMP from IMP: step 2/2.; PATHWAY: Purine metabolism; IMP biosynthesis via de novo pathway; 5-amino-1-(5-phospho-D-ribosyl)imidazole |
| Tissues Ubiquitously expressed. Both isoforms are produced by all tissues. Isoform 2 is 10-fold less abundant than isoform 1. |
| Structure Homotetramer. Residues from neighboring subunits contribute catalytic and substrate-binding residues to each active site. |
| Target Relevance information above includes information from UniProt accession: P30566 |
| The UniProt Consortium |
Data
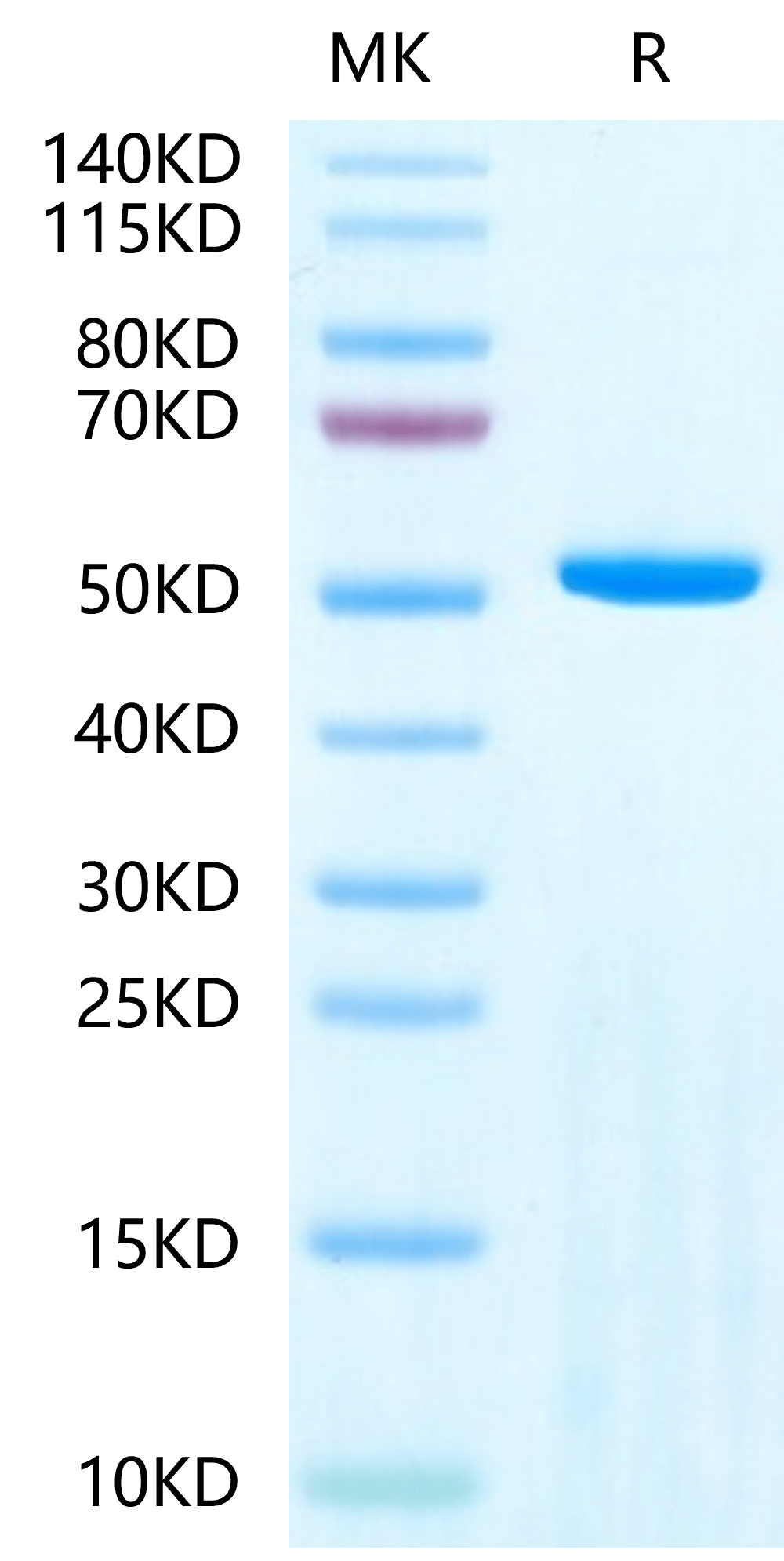 |
| Human Adenylosuccinate Lyase on Tris-Bis PAGE under reduced condition. The purity is greater than 95%. |
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||














