| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| express system | HEK293 |
| product tag | C-His-Avi |
| purity | > 95% as determined by Tris-Bis PAGE |
| background | Siglec-8, also known as SAF, is an approximately 75 kDa transmembrane glycoprotein in the Siglec family of sialic acid-binding immune regulatory molecules. Mature human Siglec-8 consists of a 347 amino acid (aa) extracellular domain (ECD) with three Ig-like domains.Putative adhesion molecule that mediates sialic-acid dependent binding to red blood cells. Preferentially binds to alpha-2, 3-linked sialic acid. Also binds to alpha-2, 6-linked sialic acid. |
| molecular weight | The protein has a predicted MW of 40.7 kDa. Due to glycosylation, the protein migrates to 50-60 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per μg by the LAL method. |
Biotinylated Human Siglec-8 Protein 4787
$525.00 – $1,750.00
Summary
- Expression: HEK293
- Pure: Yes (SDS-PAGE)
- Amino Acid Range: Met17-Ala363
Biotinylated Human Siglec-8 Protein 4787
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Siglec-8, also known as SAF, is an approximately 75 kDa transmembrane glycoprotein in the Siglec family of sialic acid-binding immune regulatory molecules. Mature human Siglec-8 consists of a 347 amino acid (aa) extracellular domain (ECD) with three Ig-like domains.Putative adhesion molecule that mediates sialic-acid dependent binding to red blood cells. Preferentially binds to alpha-2,3-linked sialic acid. Also binds to alpha-2,6-linked sialic acid. |
| Protein names Sialic acid-binding Ig-like lectin 8 (Siglec-8) (Sialoadhesin family member 2) (SAF-2) |
| Gene names SIGLEC8,SIGLEC8 SAF2 |
| Protein family Immunoglobulin superfamily, SIGLEC (sialic acid binding Ig-like lectin) family |
| Mass 9606Da |
| Function Putative adhesion molecule that mediates sialic-acid dependent binding to red blood cells (PubMed:10625619, PubMed:10856141). Preferentially binds to alpha-2,3-linked sialic acid. Also binds to alpha-2,6-linked sialic acid. The sialic acid recognition site may be masked by cis interactions with sialic acids on the same cell surface (PubMed:10625619). Recognizes simultaneously epitopes having a terminal N-acetylneuraminic acid (sialic acid) and an underlying 6-O-sulfated galactose. Preferentially binds to Gal-6-sulfated sialyl-Lewis X glycan epitopes (PubMed:27357658). |
| Catalytic activity BINDING 23; /ligand="a carbohydrate"; /ligand_id="ChEBI:CHEBI:16646"; /evidence="ECO:0000269|PubMed:27357658, ECO:0007744|PDB:2N7B"; BINDING 72..75; /ligand="a carbohydrate"; /ligand_id="ChEBI:CHEBI:16646"; /evidence="ECO:0000269|PubMed:27357658, ECO:0007744|PDB:2N7B"; BINDING 125; /ligand="a carbohydrate"; /ligand_id="ChEBI:CHEBI:16646"; /evidence="ECO:0000269|PubMed:27357658, ECO:0007744|PDB:2N7B"; BINDING 134..138; /ligand="a carbohydrate"; /ligand_id="ChEBI:CHEBI:16646"; /evidence="ECO:0000269|PubMed:27357658, ECO:0007744|PDB:2N7B" |
| Subellular location Membrane; Single-pass type I membrane protein. |
| Tissues Expressed specifically on red blood cells namely basophil, mast cells and eosinophils. |
| Domain Co |
| Target Relevance information above includes information from UniProt accession: Q9NYZ4 |
| The UniProt Consortium |
Data
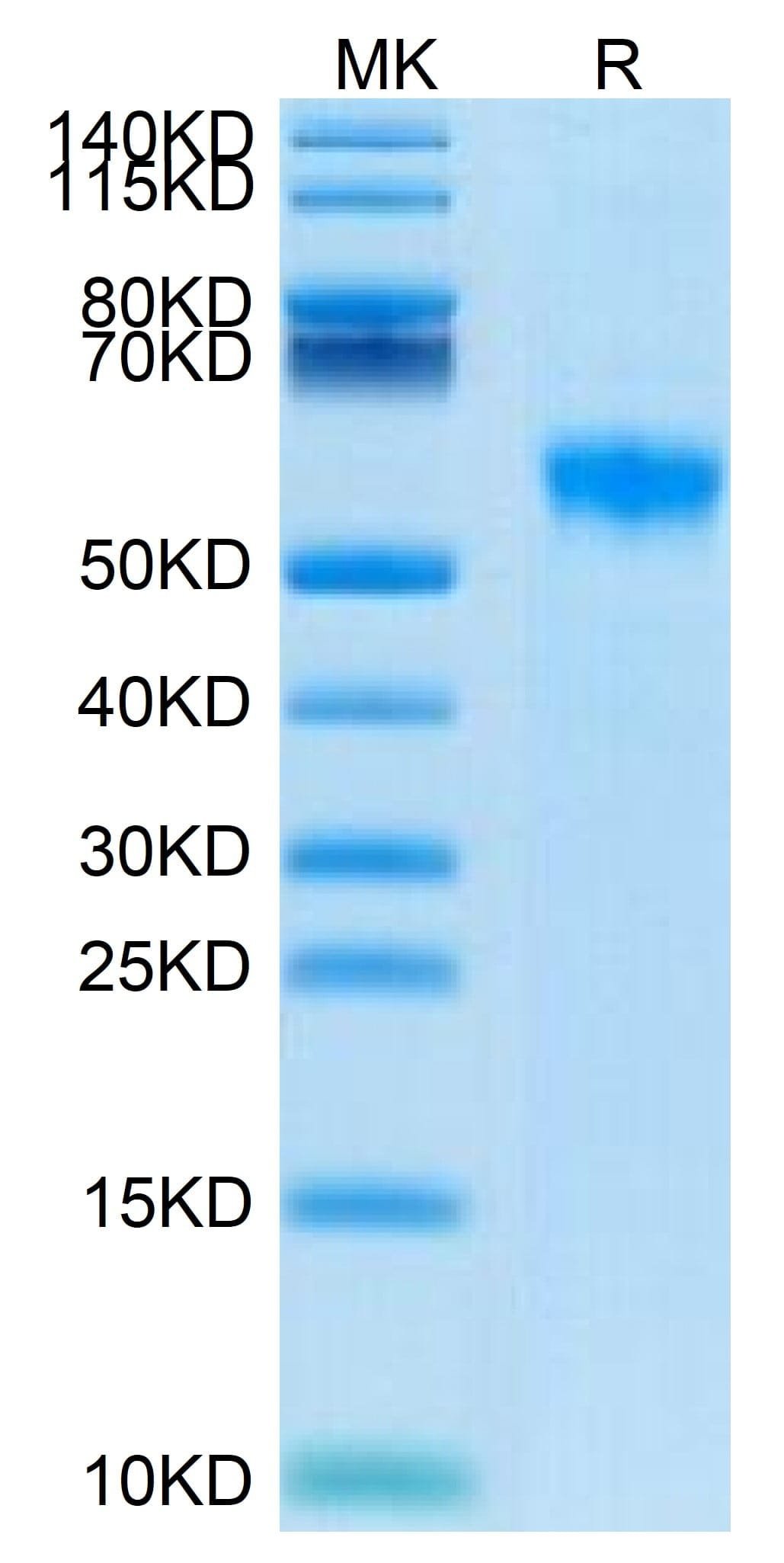 |
| Biotinylated Human Siglec-8 on Tris-Bis PAGE under reduced conditions. The purity is greater than 95%. |
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||














