| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| express system | HEK293 |
| product tag | C-hFc-Avi |
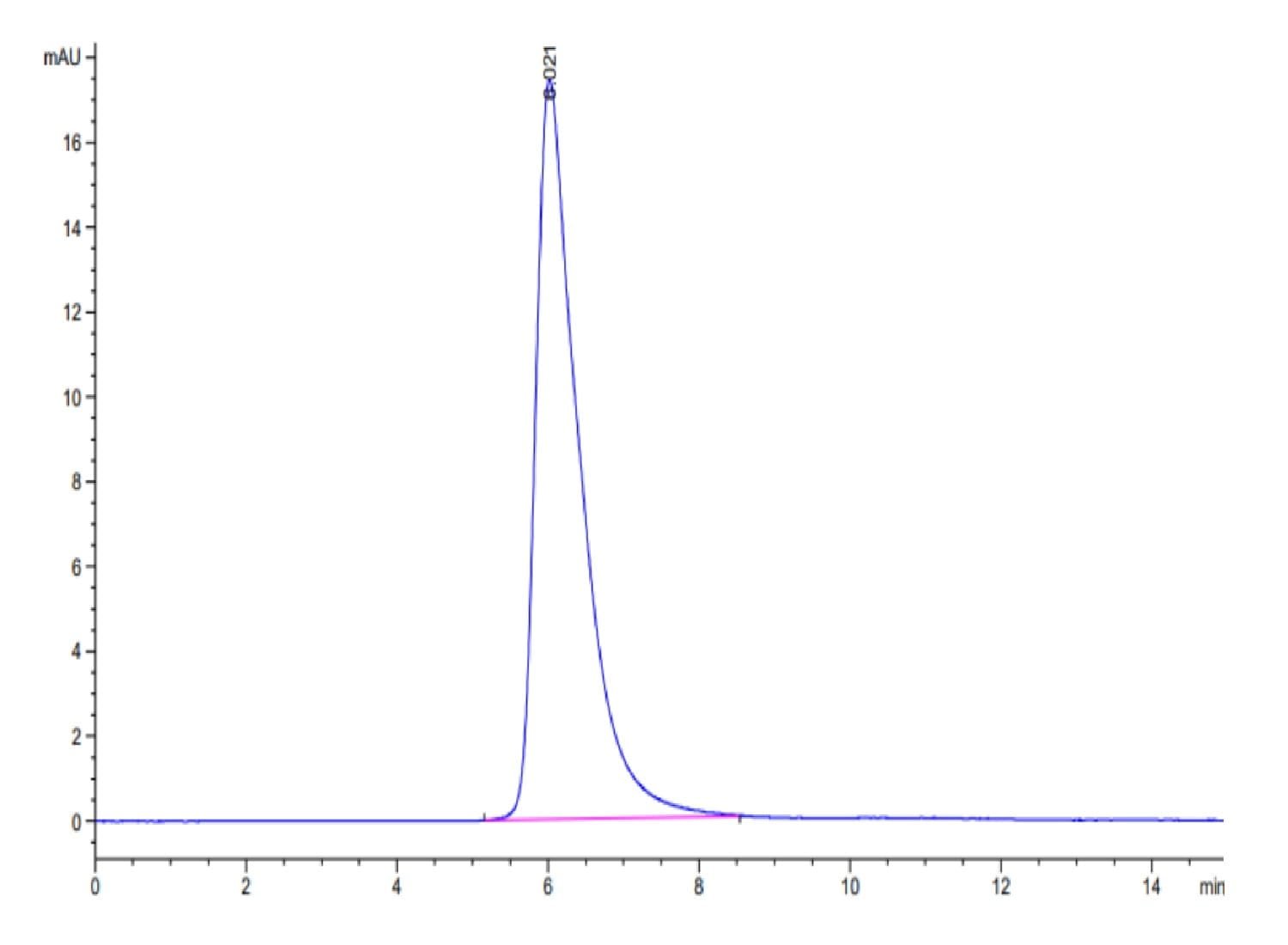
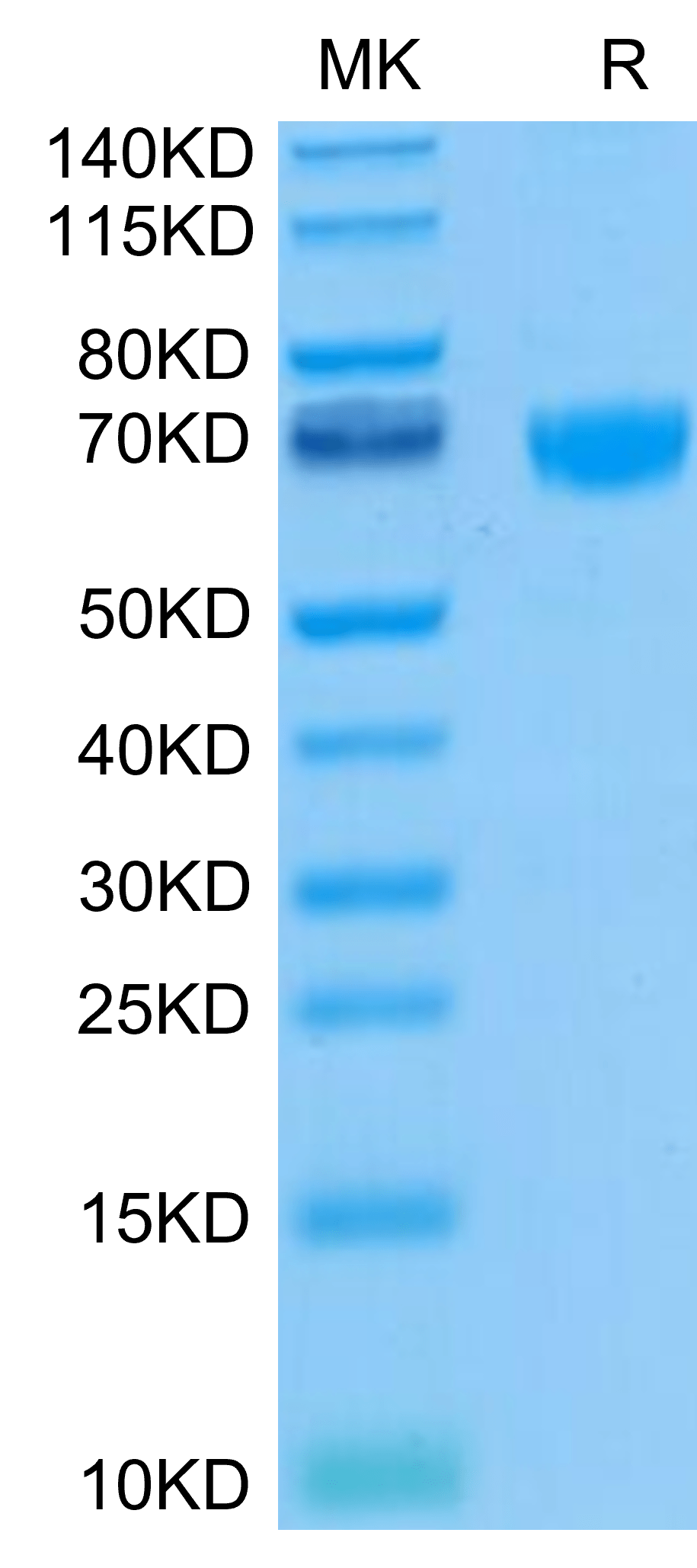
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | Programmed cell death protein 1, also known as PD-1 and CD279, is a protein found on the surface of cells that has a role in regulating the immune system's response to the cells of the human body by down-regulating the immune system and promoting self tolerance by suppressing T cell inflammatory activity. |
| molecular weight | The protein has a predicted MW of 44.7 kDa. Due to glycosylation, the protein migrates to 65-72 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per μg by the LAL method. |
Biotinylated Human PD-1/PDCD1 Protein 4697
$525.00 – $1,750.00
Summary
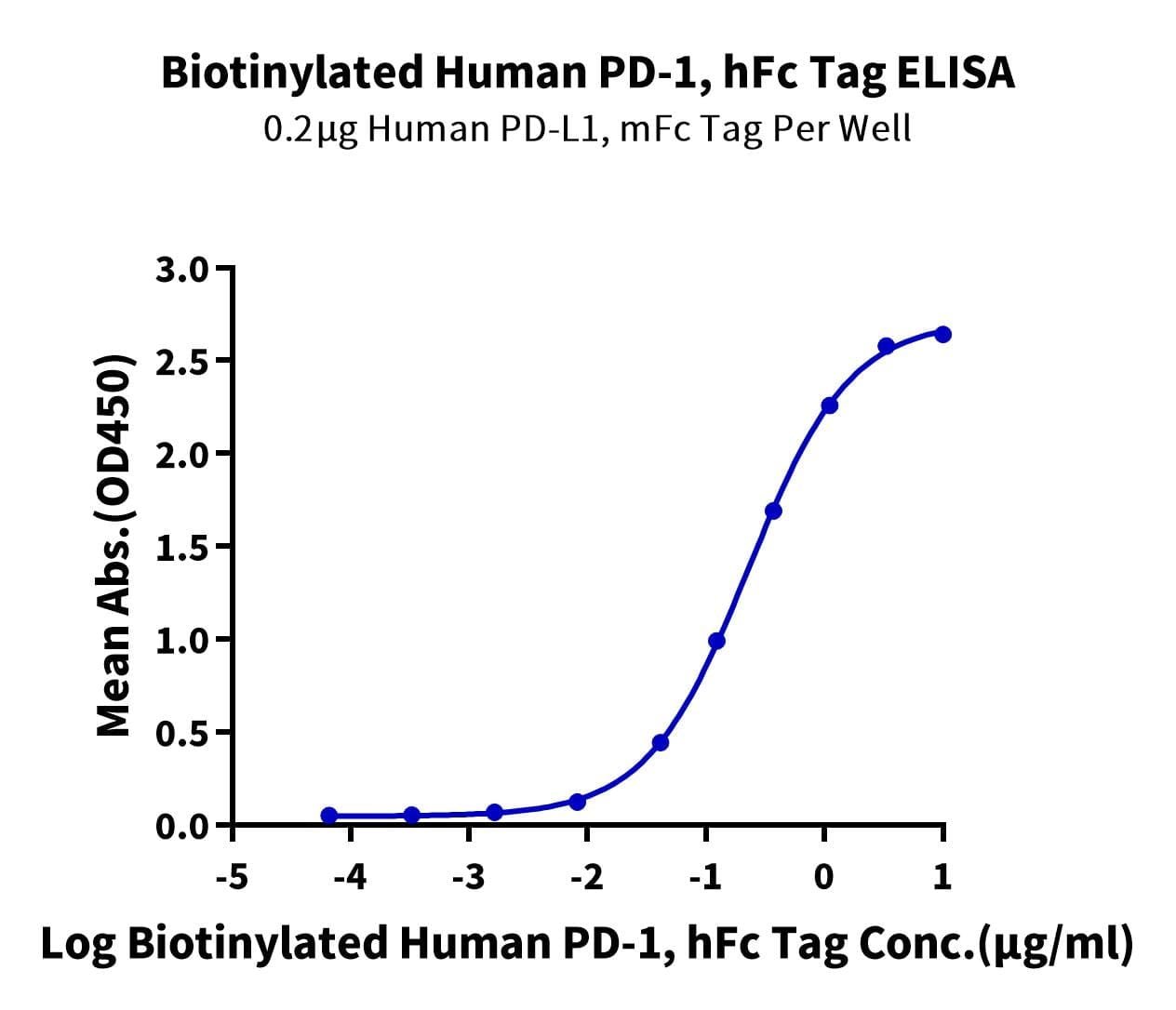
- Expression: HEK293
- Functional: Yes (ELISA)
- Amino Acid Range: Leu25-Gln167
Biotinylated Human PD-1/PDCD1 Protein 4697
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Programmed cell death protein 1, also known as PD-1 and CD279 , is a protein found on the surface of cells that has a role in regulating the immune system's response to the cells of the human body by down-regulating the immune system and promoting self tolerance by suppressing T cell inflammatory activity. |
| Protein names Programmed cell death protein 1 (Protein PD-1) (hPD-1) (CD antigen CD279) |
| Mass 31647Da |
| Function Inhibitory receptor on antigen activated T-cells that plays a critical role in induction and maintenance of immune tolerance to self (PubMed:21276005). Delivers inhibitory signals upon binding to ligands CD274/PDCD1L1 and CD273/PDCD1LG2 (PubMed:21276005). Following T-cell receptor (TCR) engagement, PDCD1 associates with CD3-TCR in the immunological synapse and directly inhibits T-cell activation (By similarity). Suppresses T-cell activation through the recruitment of PTPN11/SHP-2: following ligand-binding, PDCD1 is phosphorylated within the ITSM motif, leading to the recruitment of the protein tyrosine phosphatase PTPN11/SHP-2 that mediates dephosphorylation of key TCR proximal signaling molecules, such as ZAP70, PRKCQ/PKCtheta and CD247/CD3zeta (By similarity). {ECO:0000250|UniProtKB:Q02242, ECO:0000269|PubMed:21276005}.; The PDCD1-mediated inhibitory pathway is exploited by tumors to attenuate anti-tumor immunity and escape destruction by the immune system, thereby facilitating tumor survival (PubMed:28951311). The interaction with CD274/PDCD1L1 inhibits cytotoxic T lymphocytes (CTLs) effector function (PubMed:28951311). The blockage of the PDCD1-mediated pathway results in the reversal of the exhausted T-cell phenotype and the normalization of the anti-tumor response, providing a rationale for cancer immunotherapy (PubMed:22658127, PubMed:25034862, PubMed:25399552). {ECO:0000269|PubMed:22658127, ECO:0000269|PubMed:25034862, ECO:0000269|PubMed:25399552, ECO:0000303|PubMed:28951311}. |
| Subellular location Cell membrane {ECO:0000269|PubMed:30487606}; Single-pass type I membrane protein. |
| Structure Monomer (PubMed:26602187). Interacts with CD274/PDCD1L1 (PubMed:26602187). Interacts with CD273/PDCD1LG2 (By similarity). Interacts with FBXO38; leading to ubiquitination and degradation of PDCD1 by the proteasome (PubMed:30487606). {ECO:0000250|UniProtKB:Q02242, ECO:0000269|PubMed:26602187, ECO:0000269|PubMed:30487606}. |
| Post-translational modification Ubiquitinated at Lys-233 by the SCF(FBXO38) complex, leading to its proteasomal degradation (PubMed:30487606). Ubiquitinated via 'Lys-48'-linked polyubiquitin chains (PubMed:30487606). {ECO:0000269|PubMed:30487606}.; Tyrosine phosphorylated at Tyr-223 (within ITIM motif) and Tyr-248 (ITSM motif) upon ligand binding. Phosphorylation at Tyr-248 promotes the recruitment of the protein tyrosine phosphatase PTPN11/SHP-2 that mediates dephosphorylation of key TCR proximal signaling molecules, such as ZAP70, PRKCQ/PKCtheta and CD247/CD3zeta. {ECO:0000250|UniProtKB:Q02242}.; N-glycosylation at Asn-58 contains at least two N-acetylglucosamine units and one fucose (PubMed:28165004). N-glycosylation does not affect binding to nivolumab drug (PubMed:28165004). {ECO:0000269|PubMed:28165004}. |
| Target Relevance information above includes information from UniProt accession: Q15116 |
| The UniProt Consortium |
Data
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||