| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | P35052 |
| express system | HEK293 |
| product tag | C-His |
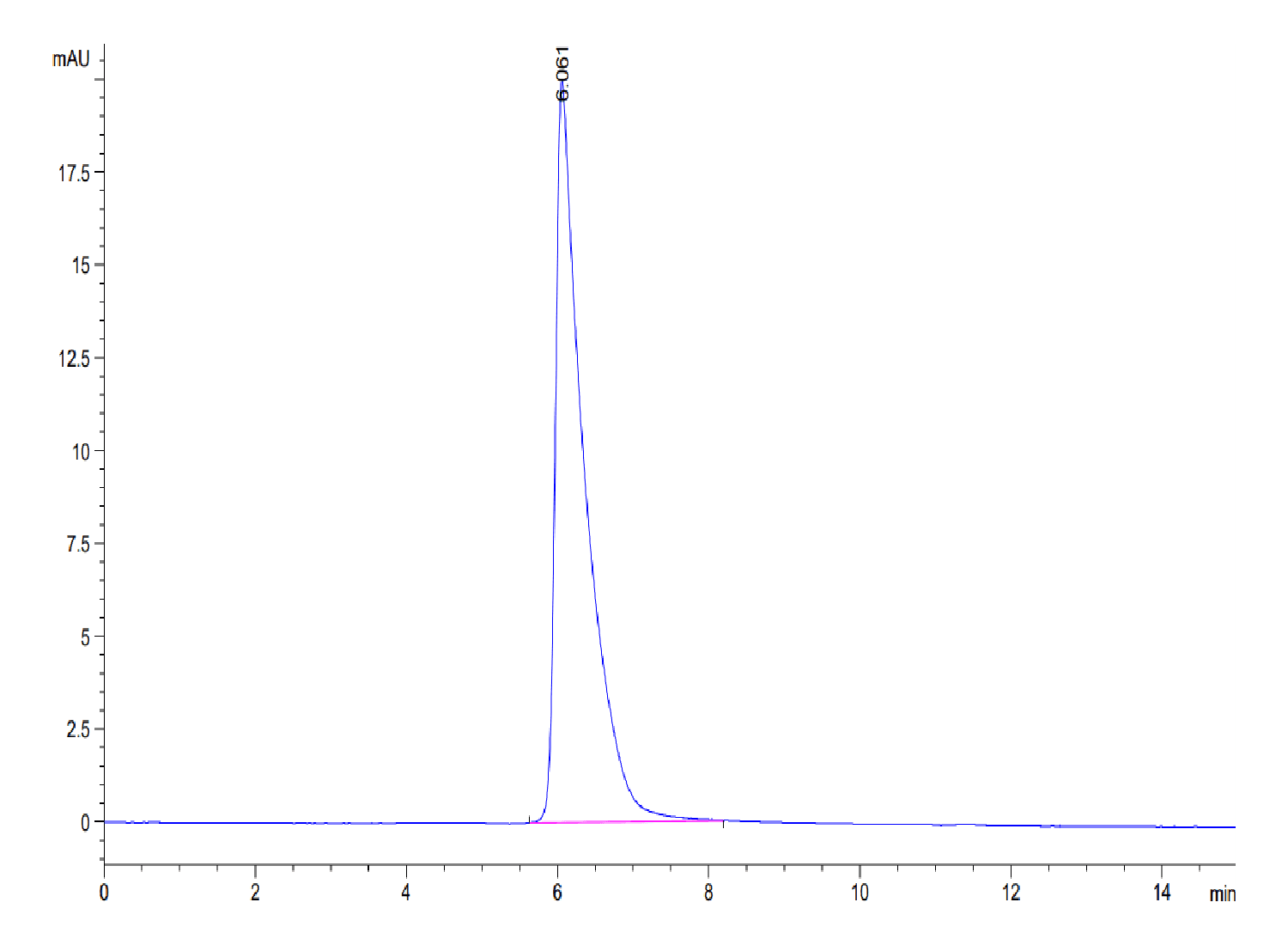
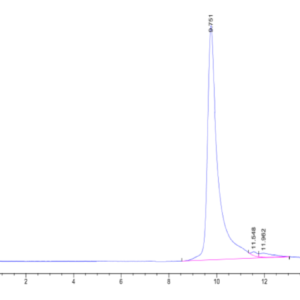
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | CAR-T cells targeting glypican-1 (GPC1)-specific human and murine CAR-T cells generated from our original anti-human/mouse GPC1 antibody showed strong antitumor effects in xenogeneic and syngeneic mouse models, respectively. Importantly, the murine CAR-T cells enhanced endogenous T cell responses against a non-GPC1 tumor antigen through the mechanism of antigen-spreading and showed synergistic antitumor effects with anti-PD-1 antibody without any adverse effects in syngeneic models. |
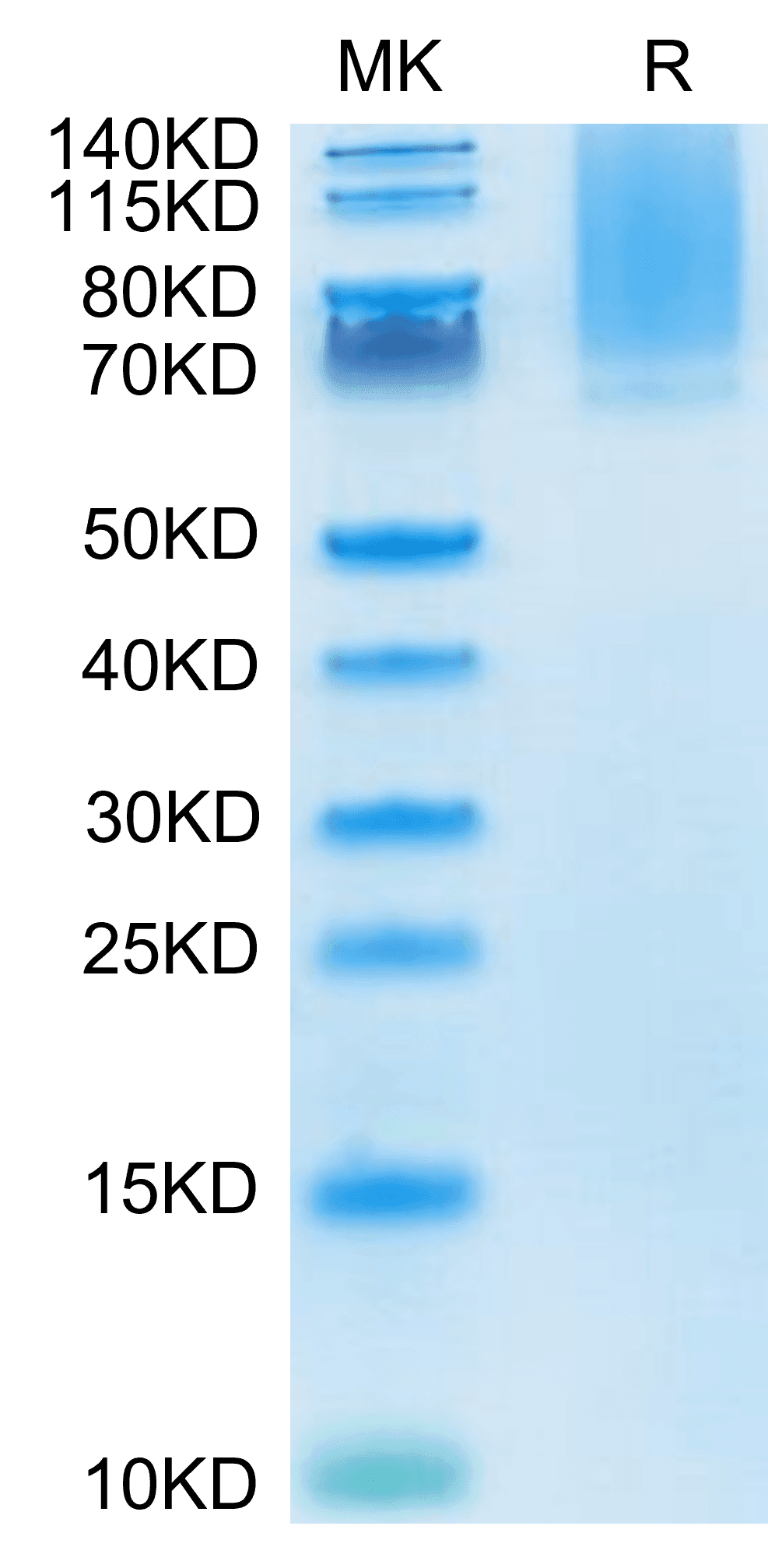
| molecular weight | The protein has a predicted MW of 57.1 kDa. Due to glycosylation, the protein migrates to 70-135 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per μg by the LAL method. |
Human Glypican 1/GPC1 Protein 4132
$315.00 – $1,050.00
Summary
- Expression: HEK293
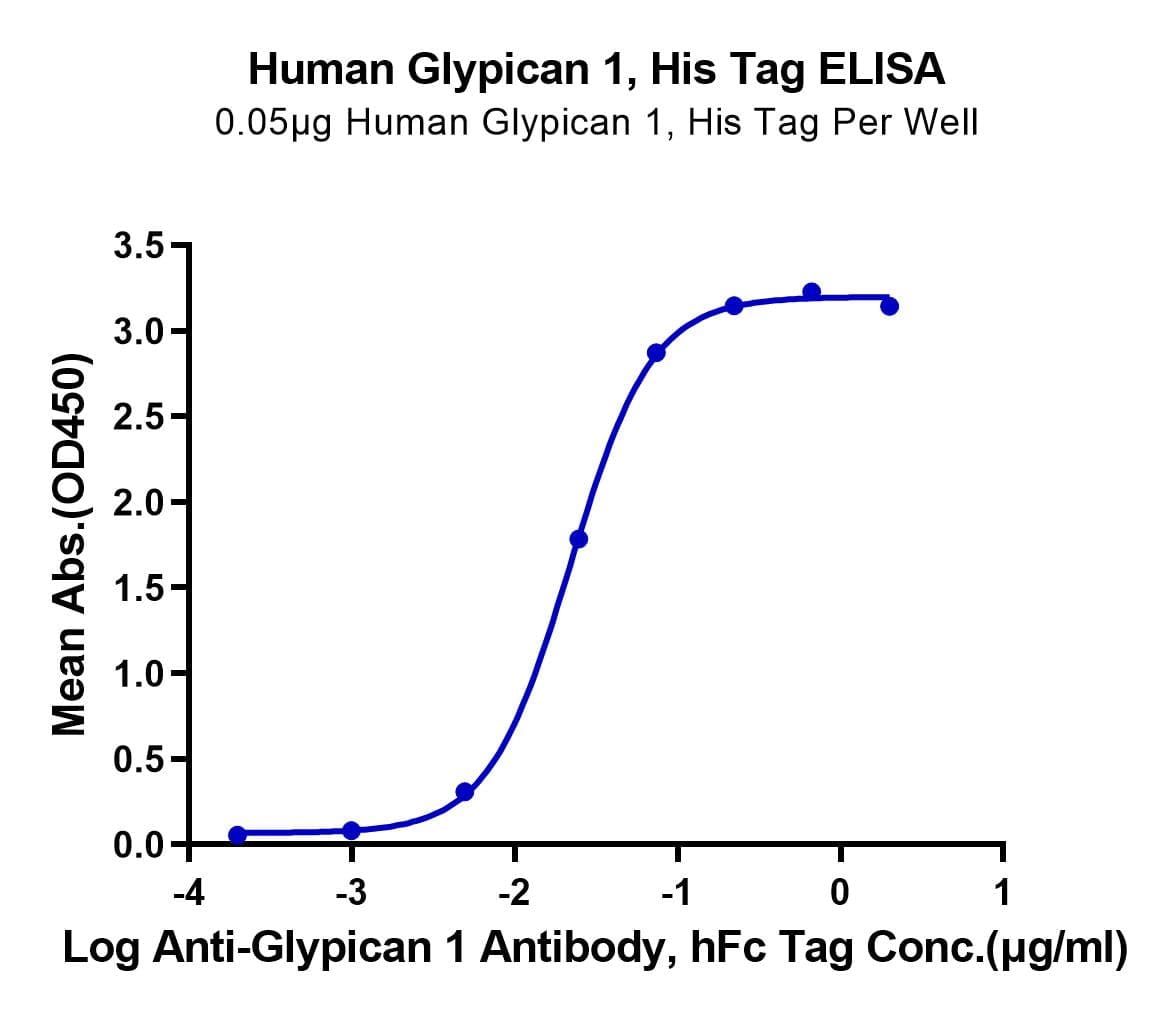
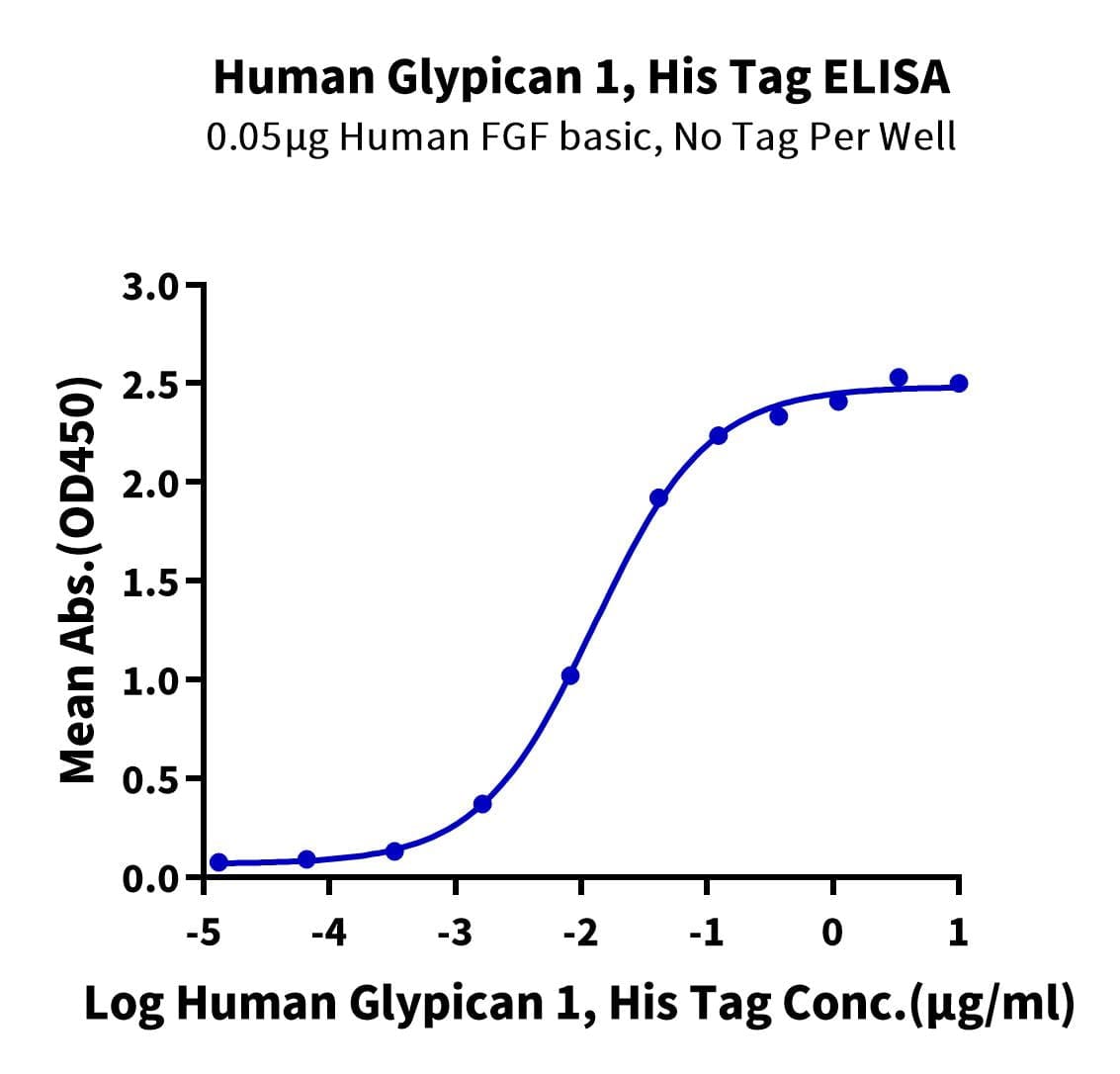
- Functional: Yes (ELISA)
- Amino Acid Range: Asp24-Ser530
Human Glypican 1/GPC1 Protein 4132
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
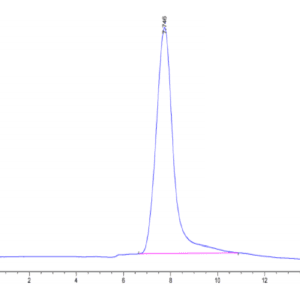
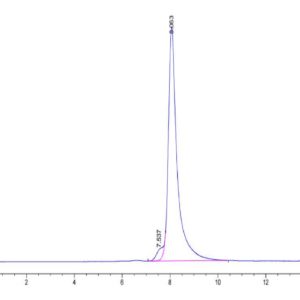
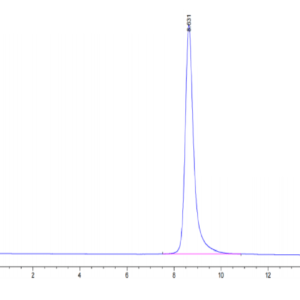
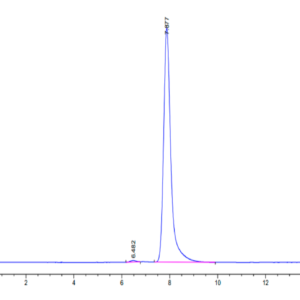
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| CAR-T cells targeting glypican-1 (GPC1)-specific human and murine CAR-T cells generated from our original anti-human/mouse GPC1 antibody showed strong antitumor effects in xenogeneic and syngeneic mouse models, respectively. Importantly, the murine CAR-T cells enhanced endogenous T cell responses against a non-GPC1 tumor antigen through the mechanism of antigen-spreading and showed synergistic antitumor effects with anti-PD-1 antibody without any adverse effects in syngeneic models. |
| Protein names Glypican-1 [Cleaved into: Secreted glypican-1] |
| Gene names GPC1,GPC1 |
| Protein family Glypican family |
| Mass 9606Da |
| Function Cell surface proteoglycan that bears heparan sulfate. Binds, via the heparan sulfate side chains, alpha-4 (V) collagen and participates in Schwann cell myelination (By similarity). May act as a catalyst in increasing the rate of conversion of prion protein PRPN(C) to PRNP(Sc) via associating (via the heparan sulfate side chains) with both forms of PRPN, targeting them to lipid rafts and facilitating their interaction. Required for proper skeletal muscle differentiation by sequestering FGF2 in lipid rafts preventing its binding to receptors (FGFRs) and inhibiting the FGF-mediated signaling. |
| Catalytic activity #N/A |
| Subellular location Cell membrane; Lipid-anchor, GPI-anchor; Extracellular side. Endosome. Note=S-nitrosylated form recycled in endosomes. Localizes to CAV1-containing vesicles close to the cell surface. Cleavage of heparan sulfate side chains takes place mainly in late endosomes. Associates with both forms of PRNP in lipid rafts. Colocalizes with APP in perinuclear compartments and with CP in intracellular compartments. Associates with fibrillar APP amyloid-beta peptides in lipid rafts in Alzheimer disease brains.; [Secreted glypican-1]: Secreted, extracellular space. |
| Post-translational modification S-nitrosylated in a Cu(2+)-dependent manner. Nitric acid (NO) is released from the nitrosylated cysteines by ascorbate or by some other reducing agent, in a Cu(2+) or Zn(2+) dependent manner. This free nitric oxide is then capable of cleaving the heparan sulfate side chains.; N- and O-glycosylated. N-glycosylation is mainly of the complex type containing sialic acid. O-glycosylated with heparan sulfate. The heparan sulfate chains can be cleaved either by the action of heparanase or, degraded by a deaminative process that uses nitric oxide (NO) released from the S-nitrosylated cysteines. This process is triggered by ascorbate, or by some other reducing agent, in a Cu(2+)- or Zn(2+) dependent manner. Cu(2+) ions are provided by ceruloproteins such as APP, PRNP or CP which associate with GCP1 in intracellular compartments or lipid rafts.; This cell-associated glypican is further processed to give rise to a medium-released species. |
| Target Relevance information above includes information from UniProt accession: P35052 |
| The UniProt Consortium |
Data
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||