| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | P98155 |
| express system | HEK293 |
| product tag | C-His |
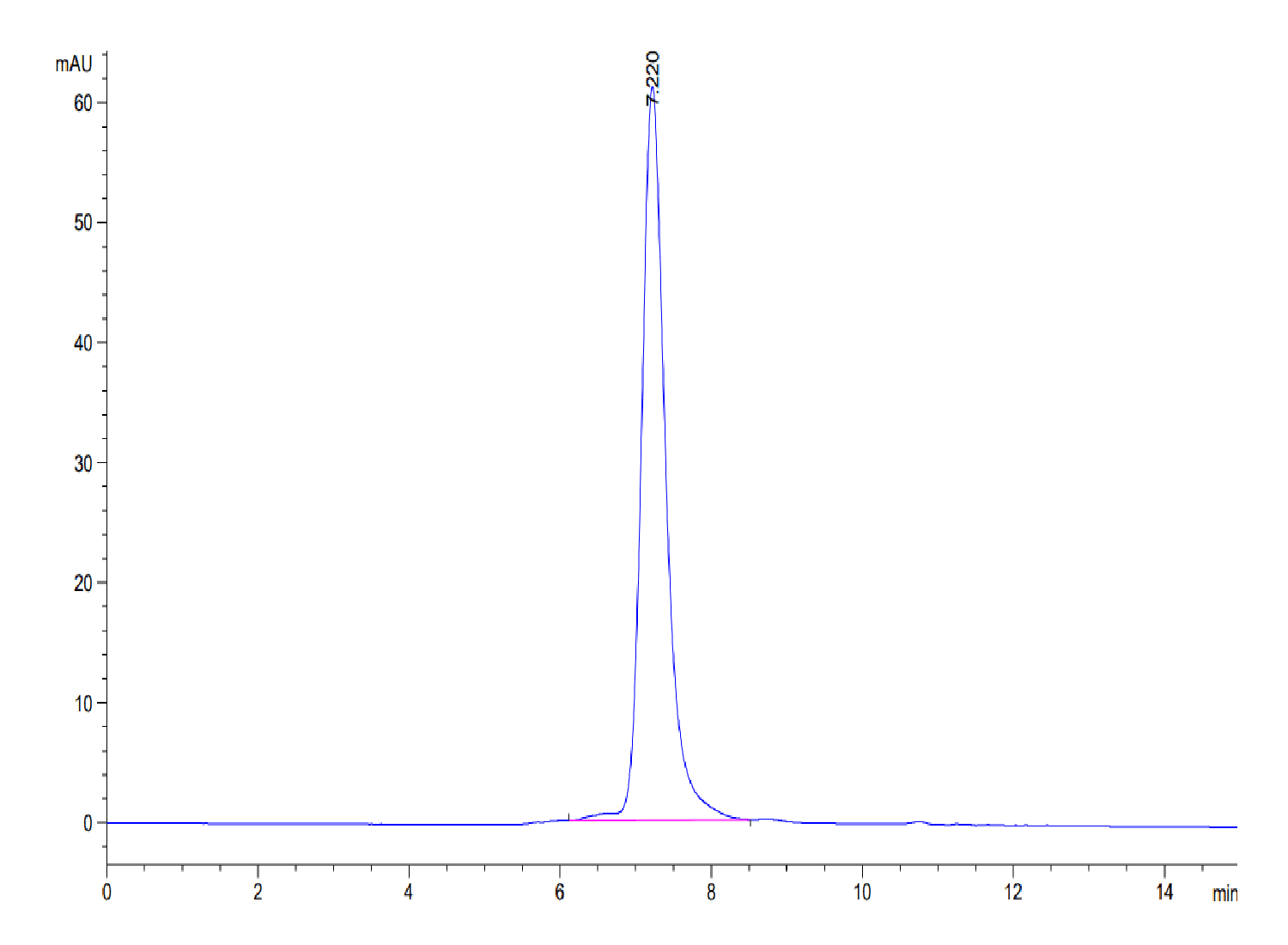
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | VLDLR cerebellar hypoplasia (VLDLR-CH) is characterized by non-progressive congenital ataxia that is predominantly truncal and results in delayed ambulation, moderate-to-profound intellectual disability, dysarthria, strabismus, and seizures.VLDLR-CH is inherited in an autosomal recessive manner. Carrier testing for at-risk relatives, prenatal testing for a pregnancy at increased risk and preimplantation genetic testing are possible when the pathogenic variants in a family are known. |
| molecular weight | The protein has a predicted MW of 85.9 kDa. Due to glycosylation, the protein migrates to 115-125 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per μg by the LAL method. |
Human VLDLR Protein 3871
$270.00 – $900.00
Summary
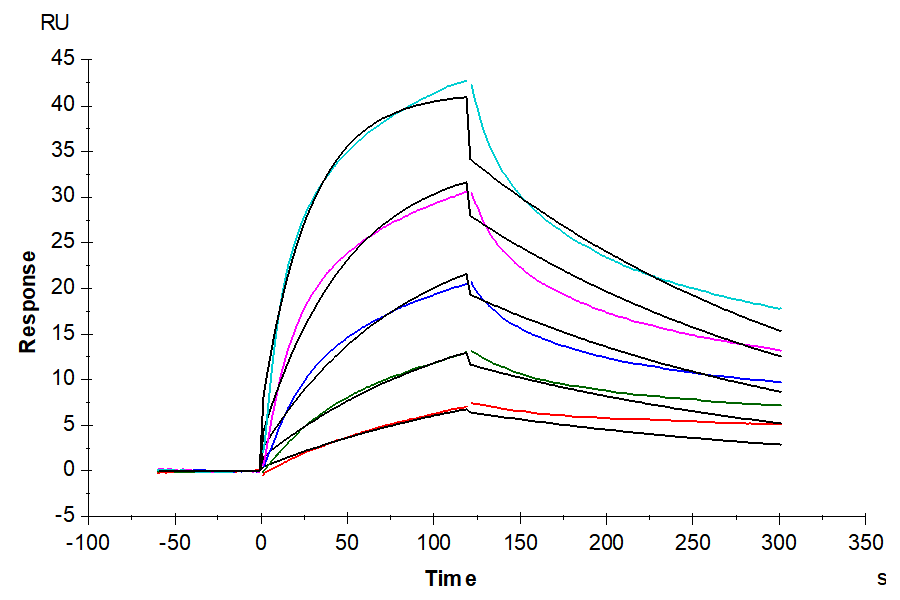
- Expression: HEK293
- Binding assay: Yes (SPR)
- Amino Acid Range: Gly28-Ser797
Human VLDLR Protein 3871
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| VLDLR cerebellar hypoplasia (VLDLR-CH) is characterized by non-progressive congenital ataxia that is predominantly truncal and results in delayed ambulation, moderate-to-profound intellectual disability, dysarthria, strabismus, and seizures.VLDLR-CH is inherited in an autosomal recessive manner. Carrier testing for at-risk relatives, prenatal testing for a pregnancy at increased risk and preimplantation genetic testing are possible when the pathogenic variants in a family are known. |
| Protein names Very low-density lipoprotein receptor (VLDL receptor) (VLDL-R) |
| Gene names VLDLR,VLDLR |
| Mass 9606Da |
| Function Multifunctional cell surface receptor that binds VLDL and transports it into cells by endocytosis and therefore plays an important role in energy metabolism. Binds also to a wide range of other molecules including Reelin/RELN or apolipoprotein E/APOE-containing ligands as well as clusterin/CLU (PubMed:24381170, PubMed:30873003). In the off-state of the pathway, forms homooligomers or heterooligomers with LRP8 (PubMed:30873003). Upon binding to ligands, homooligomers are rearranged to higher order receptor clusters that transmit the extracellular RELN signal to intracellular signaling processes by binding to DAB1 (PubMed:30873003). This interaction results in phosphorylation of DAB1 leading to the ultimate cell responses required for the correct positioning of newly generated neurons. Later, mediates a stop signal for migrating neurons, preventing them from entering the marginal zone (By similarity).; (Microbial infection) Acts as a receptor for Semliki Forest virus. |
| Catalytic activity #N/A |
| Subellular location Cell membrane ; Single-pass type I membrane protein. Membrane, clathrin-coated pit; Single-pass type I membrane protein. |
| Tissues Abundant in heart and skeletal muscle; also ovary and kidney; not in liver. |
| Structure Homooligomer (PubMed:30873003). Binds to the extracellular matrix protein Reelin/RELN (PubMed:30873003). Interacts with LRP8 (PubMed:30873003). Interacts with LDLRAP1 (By similarity). Interacts with SNX17 (By similarity). Interacts with DAB1. Interacts with PCSK9. Interacts with PAFAH1B3 and PAFAH1B2, the catalytic complex of (PAF-AH (I)) heterotetrameric enzyme; these interactions may modulate the Reelin pathway (PubMed:17330141). Interacts with STX5; this interaction mediates VLDLR translocation from the endoplasmic reticulum to the plasma membrane (PubMed:23701949). Interacts with CLU (PubMed:24381170).; (Microbial infection) Interacts with protein VP1 of the minor-group human rhinoviruses (HRVs) through the second and third LDL-receptor class A domains.; (Microbial infection) Interacts with Semliki Forest virus E2-E1 heterodimer; this interaction mediates viral entry to host cell. |
| Post-translational modification Ubiquitinated at Lys-839 by MYLIP leading to degradation.; Glycosylated. |
| Target Relevance information above includes information from UniProt accession: P98155 |
| The UniProt Consortium |
Data
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||