| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | NP_000362 |
| express system | HEK293 |
| product tag | C-His |
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | Transthyretin is a highly conserved homotetrameric protein, mainly synthetized by the liver and the choroid plexus of brain. The carrier role of TTR is well-known; however, many other functions have emerged, namely in the nervous system. TTR aggregates are responsible for many amyloidosis such as familial amyloidotic polyneuropathy and cardiomyopathy. Normal TTR can also aggregate and deposit in the heart of old people and in preeclampsia placental tissue. |
| molecular weight | The protein has a predicted MW of 14.9 kDa. Due to glycosylation, the protein migrates to 16 kDa and 39 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per μg by the LAL method. |
Human Transthyretin/Prealbumin Protein 3690
$240.00 – $800.00
Summary
- Expression: HEK293
- Pure: Yes (HPLC)
- Amino Acid Range: Gly21-Glu147
Human Transthyretin/Prealbumin Protein 3690
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Transthyretin is a highly conserved homotetrameric protein, mainly synthetized by the liver and the choroid plexus of brain. The carrier role of TTR is well-known; however, many other functions have emerged, namely in the nervous system. TTR aggregates are responsible for many amyloidosis such as familial amyloidotic polyneuropathy and cardiomyopathy. Normal TTR can also aggregate and deposit in the heart of old people and in preeclampsia placental tissue. |
| Protein names Transthyretin (ATTR) (Prealbumin) (TBPA) |
| Protein family Transthyretin family |
| Mass 15887Da |
| Function Thyroid hormone-binding protein. Probably transports thyroxine from the bloodstream to the brain. {ECO:0000269|PubMed:3714052}. |
| Subellular location Secreted. Cytoplasm. |
| Tissues Detected in serum and cerebrospinal fluid (at protein level). Highly expressed in choroid plexus epithelial cells. Detected in retina pigment epithelium and liver. {ECO:0000269|PubMed:10328977, ECO:0000269|PubMed:3714052}. |
| Structure Homotetramer. Dimer of dimers. In the homotetramer, subunits assemble around a central channel that can accommodate two ligand molecules. Interacts with RBP4. {ECO:0000269|PubMed:10052934, ECO:0000269|PubMed:11243784, ECO:0000269|PubMed:11560492, ECO:0000269|PubMed:12820260, ECO:0000269|PubMed:14583036, ECO:0000269|PubMed:14711308, ECO:0000269|PubMed:15735344, ECO:0000269|PubMed:15769474, ECO:0000269|PubMed:15826192, ECO:0000269|PubMed:18095641, ECO:0000269|PubMed:18155178, ECO:0000269|PubMed:18811132, ECO:0000269|PubMed:19021760}. |
| Post-translational modification Not glycosylated under normal conditions. Following unfolding, caused for example by variant AMYL-TTR 'Gly-38', the cryptic Asn-118 site is exposed and glycosylated by STT3B-containing OST complex, leading to its degradation by the ER-associated degradation (ERAD) pathway. {ECO:0000269|PubMed:14760718, ECO:0000269|PubMed:16335952, ECO:0000269|PubMed:19167329}.; Sulfonation of the reactive cysteine Cys-30 enhances the stability of the native conformation of TTR, avoiding misassembly of the protein leading to amyloid formation. {ECO:0000305|PubMed:17175208}. |
| Domain Each monomer has two 4-stranded beta sheets and the shape of a prolate ellipsoid. Antiparall |
| Target Relevance information above includes information from UniProt accession: P02766 |
| The UniProt Consortium |
Data
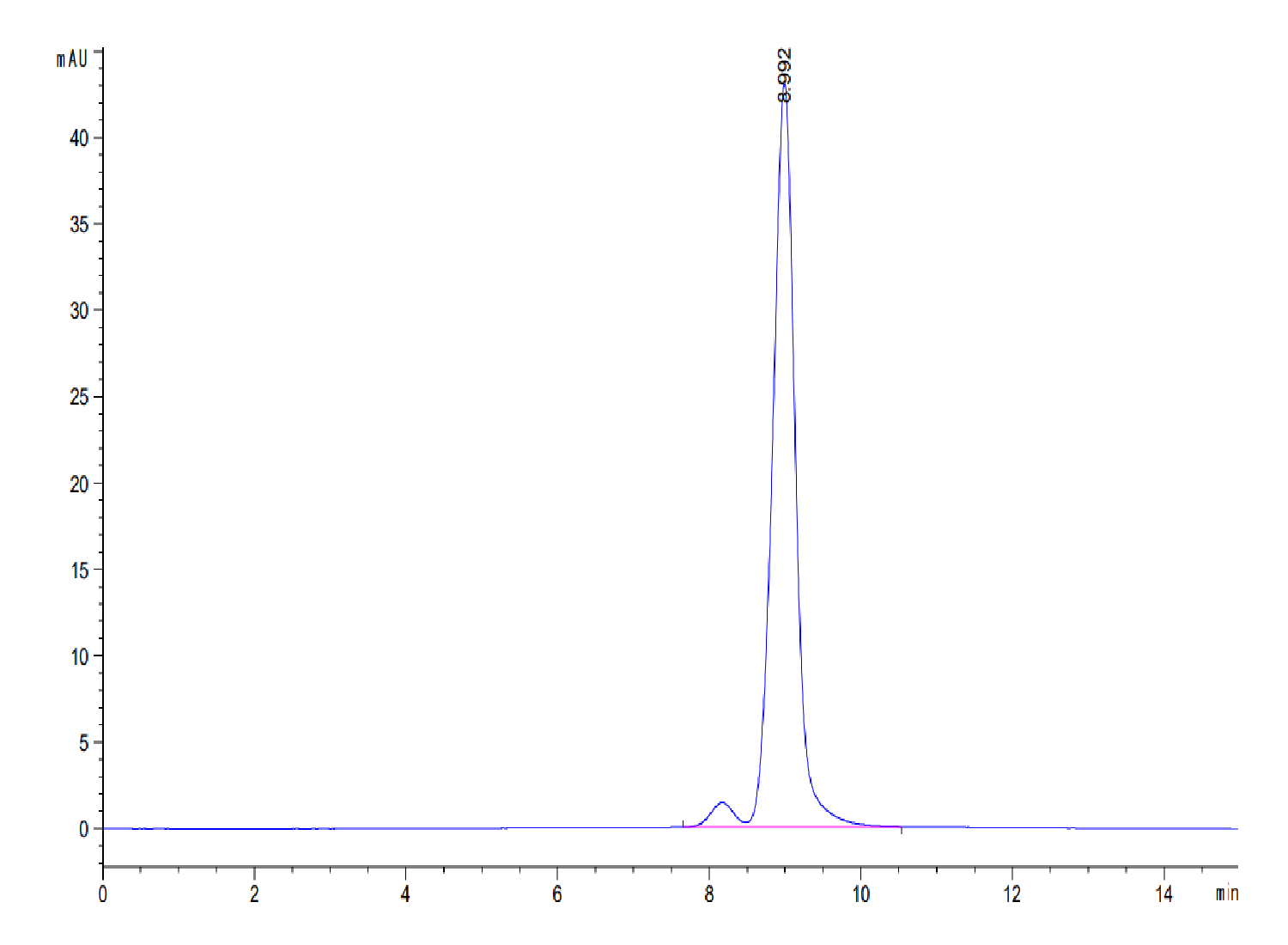 |
| The purity of Human Transthyretin/Prealbumin is greater than 95% as determined by SEC-HPLC. |
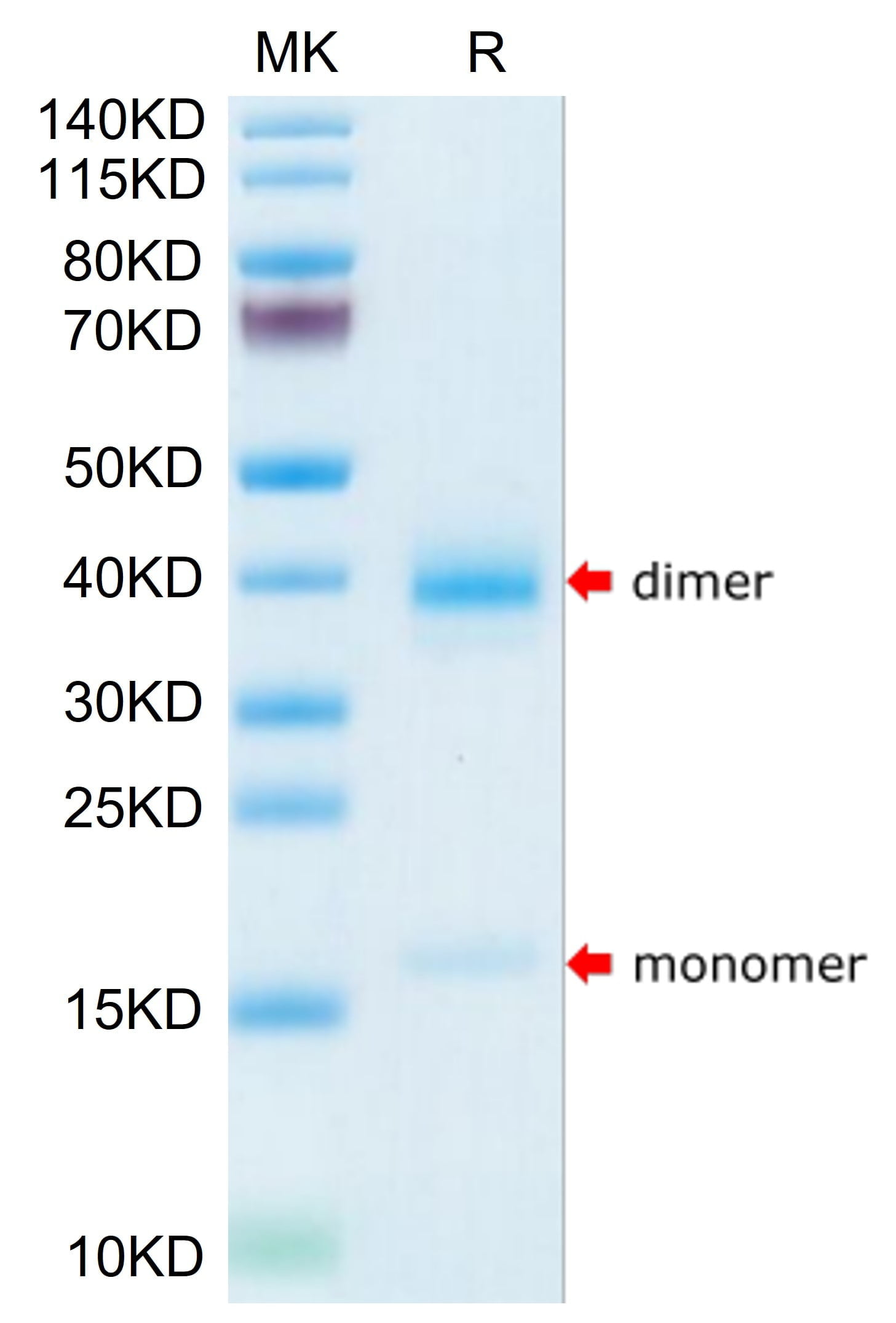 |
| Human Transthyretin/Prealbumin on Tris-Bis PAGE under reduced condition. The purity is greater than 95%. |
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||














