| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | P28798 |
| express system | HEK293 |
| product tag | C-His |
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | Haploinsufficiency of progranulin (PGRN) is a leading cause of frontotemporal lobar degeneration (FTLD). Loss of PGRN leads to lysosome dysfunction during aging. TMEM106B, a gene encoding a lysosomal membrane protein, is the main risk factor for FTLD with PGRN haploinsufficiency.Loss of both PGRN and TMEM106B results in an increased accumulation of lysosomal vacuoles in the axon initial segment of motor neurons and enhances the manifestation of FTLD phenotypes with a much earlier onset. |
| molecular weight | The protein has a predicted MW of 62.7 kDa. Due to glycosylation, the protein migrates to 70-80 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per μg by the LAL method. |
Mouse GEP Protein 3611
$270.00 – $900.00
Summary
- Expression: HEK293
- Pure: Yes (HPLC)
- Amino Acid Range: Thr18-Leu589
Mouse GEP Protein 3611
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Haploinsufficiency of progranulin (PGRN) is a leading cause of frontotemporal lobar degeneration (FTLD). Loss of PGRN leads to lysosome dysfunction during aging. TMEM106B, a gene encoding a lysosomal membrane protein, is the main risk factor for FTLD with PGRN haploinsufficiency.Loss of both PGRN and TMEM106B results in an increased accumulation of lysosomal vacuoles in the axon initial segment of motor neurons and enhances the manifestation of FTLD phenotypes with a much earlier onset. |
| Protein names Progranulin (PGRN) (Acrogranin) (Epithelin/granulin precursor) (Glycoprotein of 88 Kda) (GP88) (Glycoprotein 88) (PC cell-derived growth factor) (PCDGF) (Proepithelin) (PEPI) [Cleaved into: Paragranulin; Granulin-1; Granulin-2; Granulin-3; Granulin-4; Granulin-5; Granulin-6; Granulin-7] |
| Gene names Grn,Grn |
| Protein family Granulin family |
| Mass 10090Da |
| Function Secreted protein that acts as a key regulator of lysosomal function and as a growth factor involved in inflammation, wound healing and cell proliferation (PubMed:12524533, PubMed:20026663, PubMed:23041626, PubMed:27789271, PubMed:28073925, PubMed:28453791, PubMed:28541286, PubMed:8496151). Regulates protein trafficking to lysosomes and, also the activity of lysosomal enzymes (PubMed:27789271, PubMed:28453791, PubMed:28541286). Facilitates also the acidification of lysosomes, causing degradation of mature CTSD by CTSB (PubMed:28073925). In addition, functions as a wound-related growth factor that acts directly on dermal fibroblasts and endothelial cells to promote division, migration and the formation of capillary-like tubule structures (PubMed:12524533). Also promotes epithelial cell proliferation by blocking TNF-mediated neutrophil activation preventing release of oxidants and proteases (PubMed:8496151). Moreover, modulates inflammation in neurons by preserving neurons survival, axonal outgrowth and neuronal integrity (PubMed:20026663, PubMed:23041626).; [Granulin-3]: Inhibits epithelial cell proliferation and induces epithelial cells to secrete IL-8.; [Granulin-7]: Stabilizes CTSD through interaction with CTSD leading to maintain its aspartic-type peptidase activity. |
| Subellular location Secreted. Lysosome. Note=Endocytosed by SORT1 and delivred to lysosomes. Targeted to lysosome by PSAP via M6PR and LRP1, in both biosynthetic and endocytic pathways (By similarity). Co-localized with GBA1 in the intracellular trafficking compartments until to lysosome (PubMed:27789271). |
| Tissues Highly expressed at the wound site and diminishes away from the wound. Not expressed in fibroblasts and endothelial cells in intact skin (PubMed:12524533). In adult brain, expressed primarily in neurons and in resting and reactive microglia (PubMed:23041626). Expressed in both neurons and microglia. Highly expressed in activated microglia in response to injury (PubMed:28541286). Expressed in macrophage (PubMed:27789271). |
| Structure Progranulin is secreted as a homodimer (By similarity). Interacts with SLPI; interaction protects progranulin from proteolysis (PubMed:12526812). Interacts (via region corresponding to granulin-7 peptide) with CTSD; stabilizes CTSD and increases its proteolytic activity. Interacts (via region corresponding to granulin-7 peptide) with SORT1; this interaction mediates endocytosis and lysosome delivery of progranulin; interaction occurs at the neuronal cell surface in a stressed nervous system (By similarity). Interacts with PSAP; facilitates lysosomal delivery of progranulin from the extracellular space and the biosynthetic pathway (PubMed:26370502). Forms a complex with PSAP and M6PR; PSAP bridges the binding between progranulin and M6PR. Forms a complex with PSAP and SORT1; progranulin bridges the interaction between PSAP and SORT1; facilitates lysosomal targeting of PSAP via SORT1; interaction enhances PSAP uptake in primary cortical neurons (By similarity). Interacts (via regions corresponding to granulin-2 and granulin-7 peptides) with GBA1; this interaction prevents aggregation of GBA1-SCARB2 complex via interaction with HSPA1A upon stress (PubMed:27789271). Interacts (via region corresponding to granulin-7 peptide) with HSPA1A; mediates recruitment of HSPA1A to GBA1 and prevents GBA1 aggregation in response to stress (By similarity). |
| Post-translational modification N-glycosylated.; Cleaved by ELANE; proteolysis is blocked by SLPI and is concentration- and time-dependent and induces CXCL8/IL-8 production; granulin-3 and granulin-4 are resistant to ELANE (By similarity). Cleaved by CTSL in lysosome thus regulating the maturation and turnover of progranulin within the lysosome (PubMed:28835281). |
| Target Relevance information above includes information from UniProt accession: P28798 |
| The UniProt Consortium |
Data
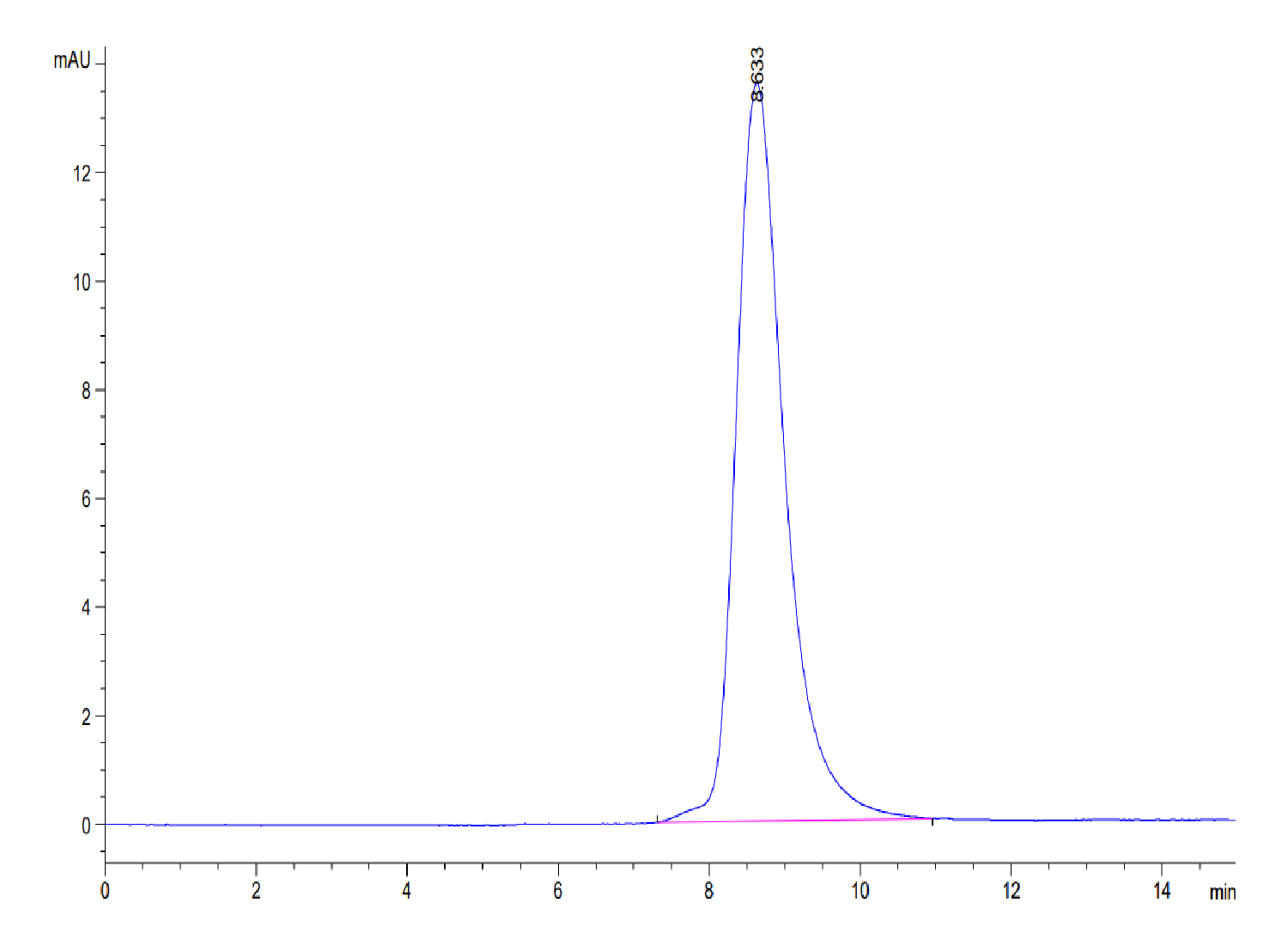 |
| The purity of Mouse GEP is greater than 95% as determined by SEC-HPLC. |
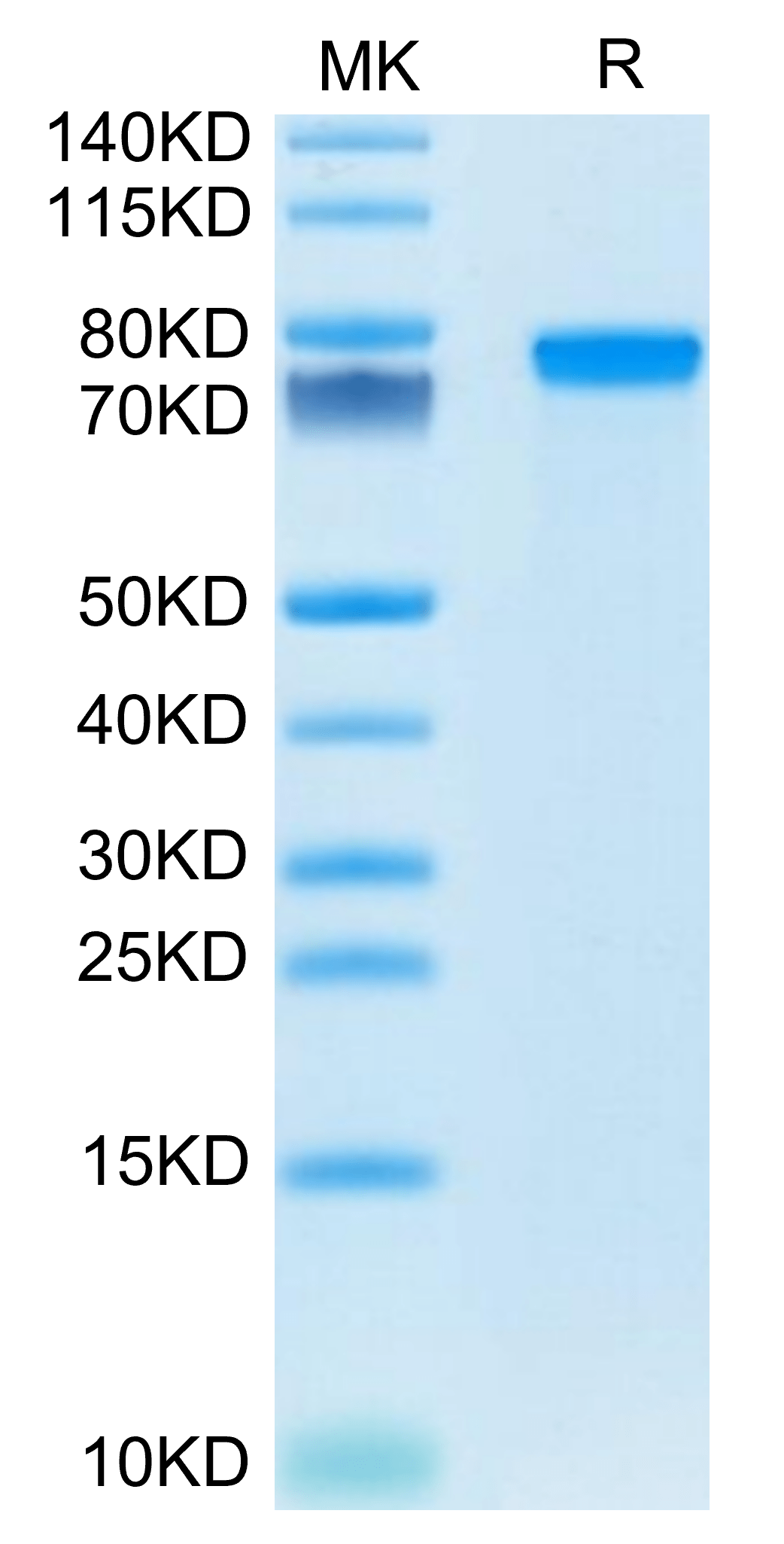 |
| Mouse GEP on Tris-Bis PAGE under reduced condition. The purity is greater than 95%. |
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||














