| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | Q99NH8 |
| express system | HEK293 |
| product tag | C-His |
| purity | > 95% as determined by Tris-Bis PAGE |
| background | TREM-2 (Triggering Receptor Expressed on Myeloid cells-2) is a 35 kDa type I transmembrane member of the TREM family and Ig superfamily. Mature human TREM-2 consists of a 156 amino acid (aa) extracellular domain (ECD) with one V-type Ig-like domain, a 21 aa transmembrane (TM) domain, and a 35 aa cytoplasmic tail. TREM-2 forms a receptor signaling complex with TYROBP which mediates signaling and cell activation following ligand binding (PubMed:10799849). Acts as a receptor for amyloid-beta protein 42, a cleavage product of the amyloid-beta precursor protein APP, and mediates its uptake and degradation by microglia. |
| molecular weight | The protein has a predicted MW of 17.9 kDa. Due to glycosylation, the protein migrates to 35-45 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per ug by the LAL method. |
Mouse TREM2 Protein 3462
$315.00 – $1,050.00
Summary
- Expression: HEK293
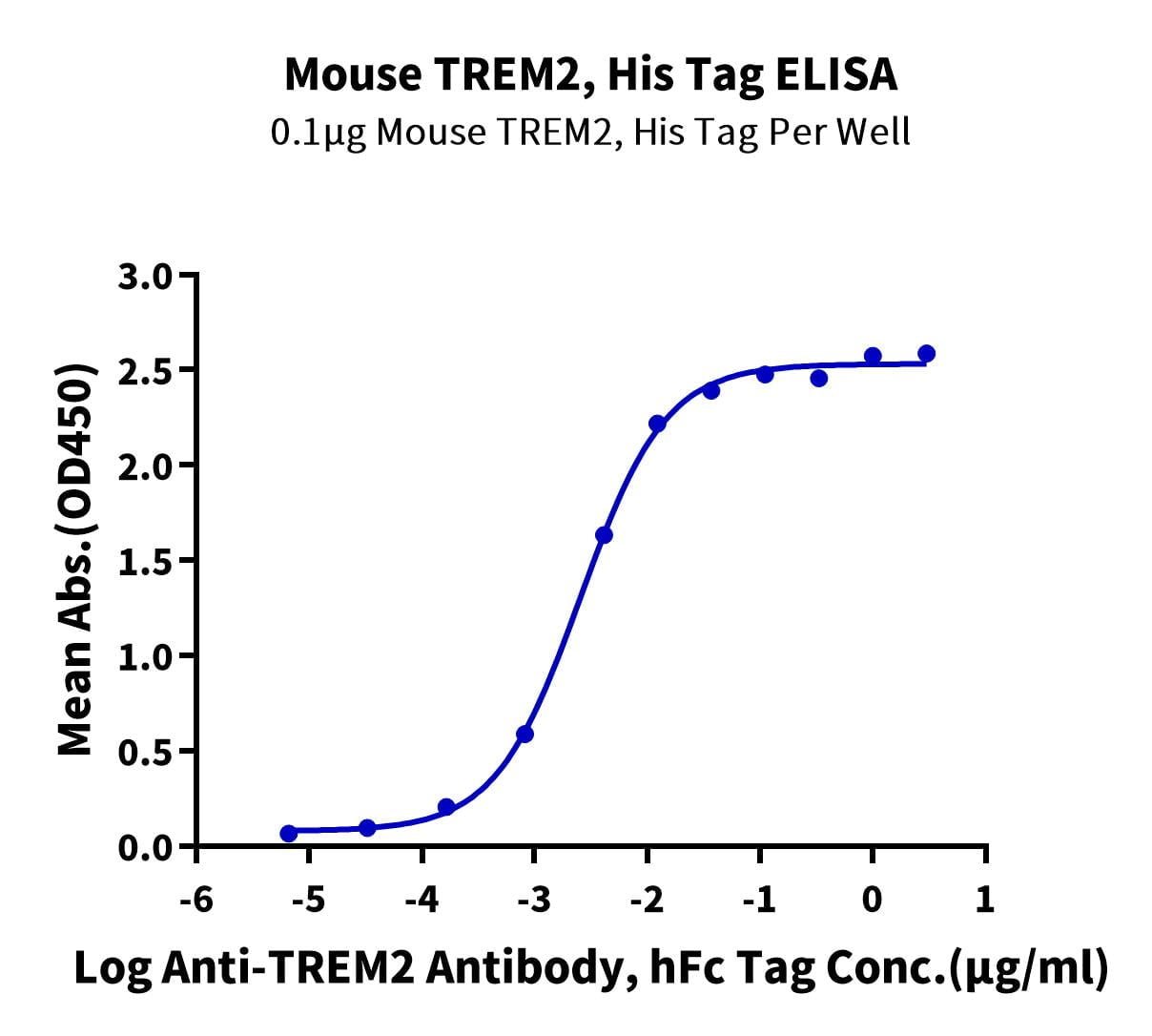
- Functional: Yes (ELISA)
- Amino Acid Range: Leu19-Ser171
Mouse TREM2 Protein 3462
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| TREM-2 (Triggering Receptor Expressed on Myeloid cells-2) is a 35 kDa type I transmembrane member of the TREM family and Ig superfamily. Mature human TREM-2 consists of a 156 amino acid (aa) extracellular domain (ECD) with one V-type Ig-like domain, a 21 aa transmembrane (TM) domain, and a 35 aa cytoplasmic tail. TREM-2 forms a receptor signaling complex with TYROBP which mediates signaling and cell activation following ligand binding (PubMed:10799849). Acts as a receptor for amyloid-beta protein 42, a cleavage product of the amyloid-beta precursor protein APP, and mediates its uptake and degradation by microglia. |
| Protein names Triggering receptor expressed on myeloid cells 2 (TREM-2) (Triggering receptor expressed on monocytes 2) |
| Gene names Trem2,Trem2 Trem2a Trem2b Trem2c |
| Mass 10090Da |
| Function Forms a receptor signaling complex with TYROBP which mediates signaling and cell activation following ligand binding (PubMed:11241283). Acts as a receptor for amyloid-beta protein 42, a cleavage product of the amyloid-beta precursor protein APP, and mediates its uptake and degradation by microglia (PubMed:27477018, PubMed:29518356). Binding to amyloid-beta 42 mediates microglial activation, proliferation, migration, apoptosis and expression of pro-inflammatory cytokines, such as IL6R and CCL3, and the anti-inflammatory cytokine ARG1 (PubMed:27477018, PubMed:29518356). Acts as a receptor for lipoprotein particles such as LDL, VLDL, and HDL and for apolipoproteins such as APOA1, APOA2, APOB, APOE, APOE2, APOE3, APOE4, and CLU and enhances their uptake in microglia (PubMed:27477018). Binds phospholipids (preferably anionic lipids) such as phosphatidylserine, phosphatidylethanolamine, phosphatidylglycerol and sphingomyelin (By similarity). Regulates microglial proliferation by acting as an upstream regulator of the Wnt/beta-catenin signaling cascade (PubMed:28077724). Required for microglial phagocytosis of apoptotic neurons (PubMed:24990881). Also required for microglial activation and phagocytosis of myelin debris after neuronal injury and of neuronal synapses during synapse elimination in the developing brain (PubMed:15728241, PubMed:25631124, PubMed:28592261, PubMed:29752066). Regulates microglial chemotaxis and process outgrowth, and also the microglial response to oxidative stress and lipopolysaccharide (PubMed:28483841, PubMed:29663649, PubMed:29859094, PubMed:30232263). It suppresses PI3K and NF-kappa-B signaling in response to lipopolysaccharide; thus promoting phagocytosis, suppressing pro-inflammatory cytokine and nitric oxide production, inhibiting apoptosis and increasing expression of IL10 and TGFB (PubMed:29663649). During oxidative stress, it promotes anti-apoptotic NF-kappa-B signaling and ERK signaling (PubMed:28592261). Plays a role in microglial MTOR activation and metabolism (PubMed:28802038). Regulates age-related changes in microglial numbers (PubMed:25631124, PubMed:29752066, PubMed:30548312). Triggers activation of the immune responses in macrophages and dendritic cells (By similarity). Mediates cytokine-induced formation of multinucleated giant cells which are formed by the fusion of macrophages (PubMed:18957693). In dendritic cells, it mediates up-regulation of chemokine receptor CCR7 and dendritic cell maturation and survival (By similarity). Involved in the positive regulation of osteoclast differentiation (PubMed:16418779). |
| Catalytic activity #N/A |
| Subellular location [Isoform 1]: Cell membrane ; Single-pass type I membrane protein .; [Isoform 2]: Secreted . |
| Tissues Expressed in the brain, specifically in microglia (at protein level) (PubMed:15728241, PubMed:27477018, PubMed:28077724, PubMed:28559417, PubMed:28592261, PubMed:28802038, PubMed:28855301, PubMed:29752066, PubMed:29794134). Expressed in macrophages (at protein level) (PubMed:11241283, PubMed:28559417, PubMed:28802038). Expressed at higher levels in the CNS, heart and lung than in lymph nodes or in other non-lymphoid tissues such as kidney, liver and testis (PubMed:12472885). In the CNS not all microglia express TREM2 (PubMed:12472885). Brain regions with an incomplete blood-brain barrier had the lowest percentages of TREM2 expressing microglia, whereas the lateral entorhinal and cingulate cortex had the highest percentages (PubMed:12472885). |
| Structure Monomer (By similarity). After ectodomain shedding, the extracellular domain oligomerizes, which is enhanced and stabilized by binding of phosphatidylserine (By similarity). Interacts with TYROBP/DAP12 (PubMed:11241283, PubMed:29518356). Interaction with TYROBP is required for stabilization of the TREM2 C-terminal fragment (TREM2-CTF) which is produced by proteolytic processing (By similarity). |
| Post-translational modification Undergoes ectodomain shedding through proteolytic cleavage by ADAM10 and ADAM17 to produce a transmembrane segment, the TREM2 C-terminal fragment (TREM2-CTF), which is subsequently cleaved by gamma-secretase. |
| Target Relevance information above includes information from UniProt accession: Q99NH8 |
| The UniProt Consortium |
Data
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||