| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| express system | HEK293 |
| product tag | C-His-Avi |
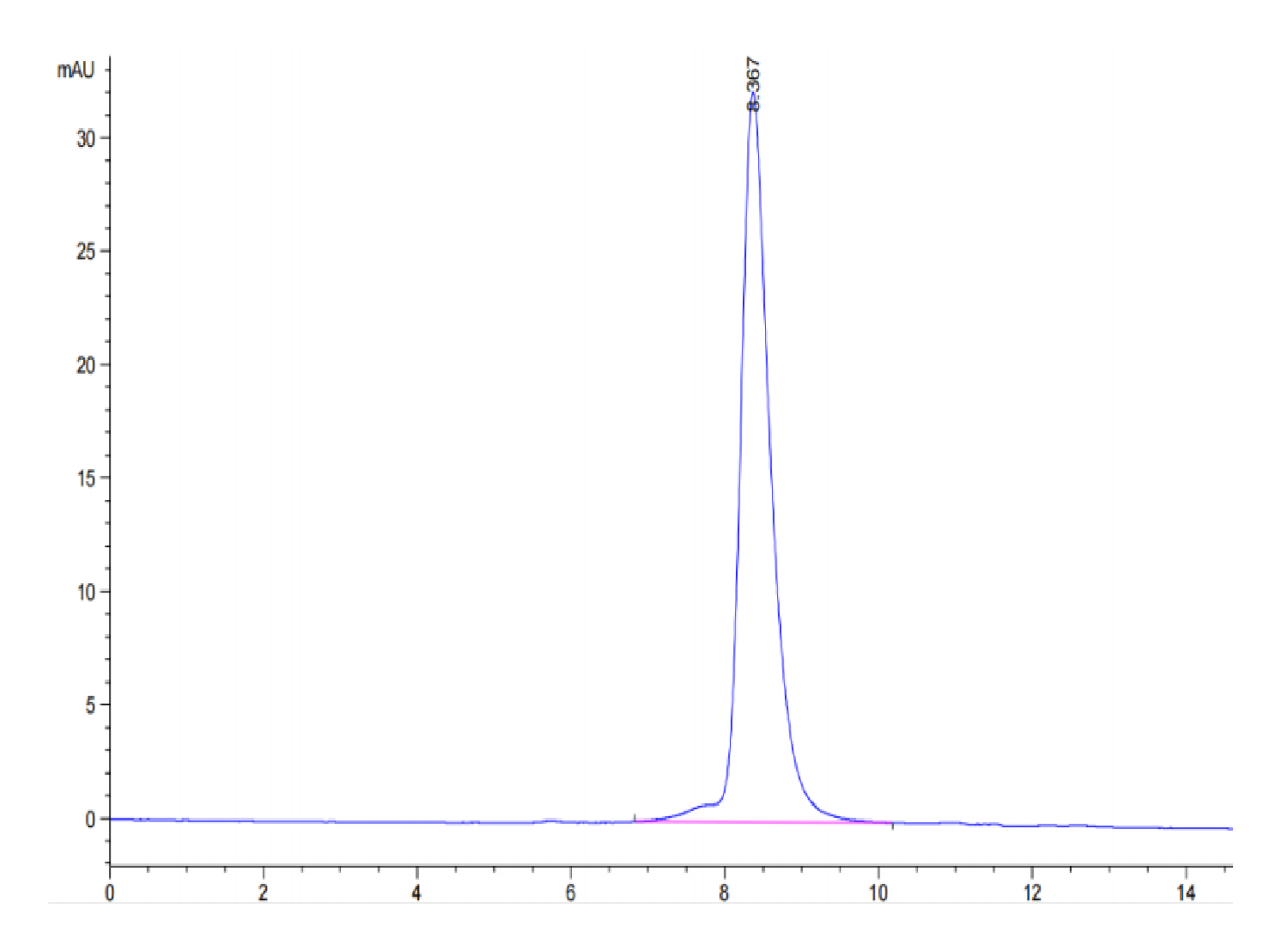
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | Guanylyl cyclase C (GUCY2C) has canonical centrality in defense of key intestinal homeostatic mechanisms, encompassing fluid and electrolyte balance, epithelial dynamics, antitumorigenesis, and intestinal barrier function. GUCY2C may represent a new target for anti-obesity pharmacotherapy. |
| molecular weight | The protein has a predicted MW of 48.8 kDa. Due to glycosylation, the protein migrates to 55-65 kDa based on Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per μg by the LAL method. |
Biotinylated Human GUCY2C/Guanylyl cyclase C Protein 3381
$525.00 – $1,750.00
Summary
- Expression: HEK293
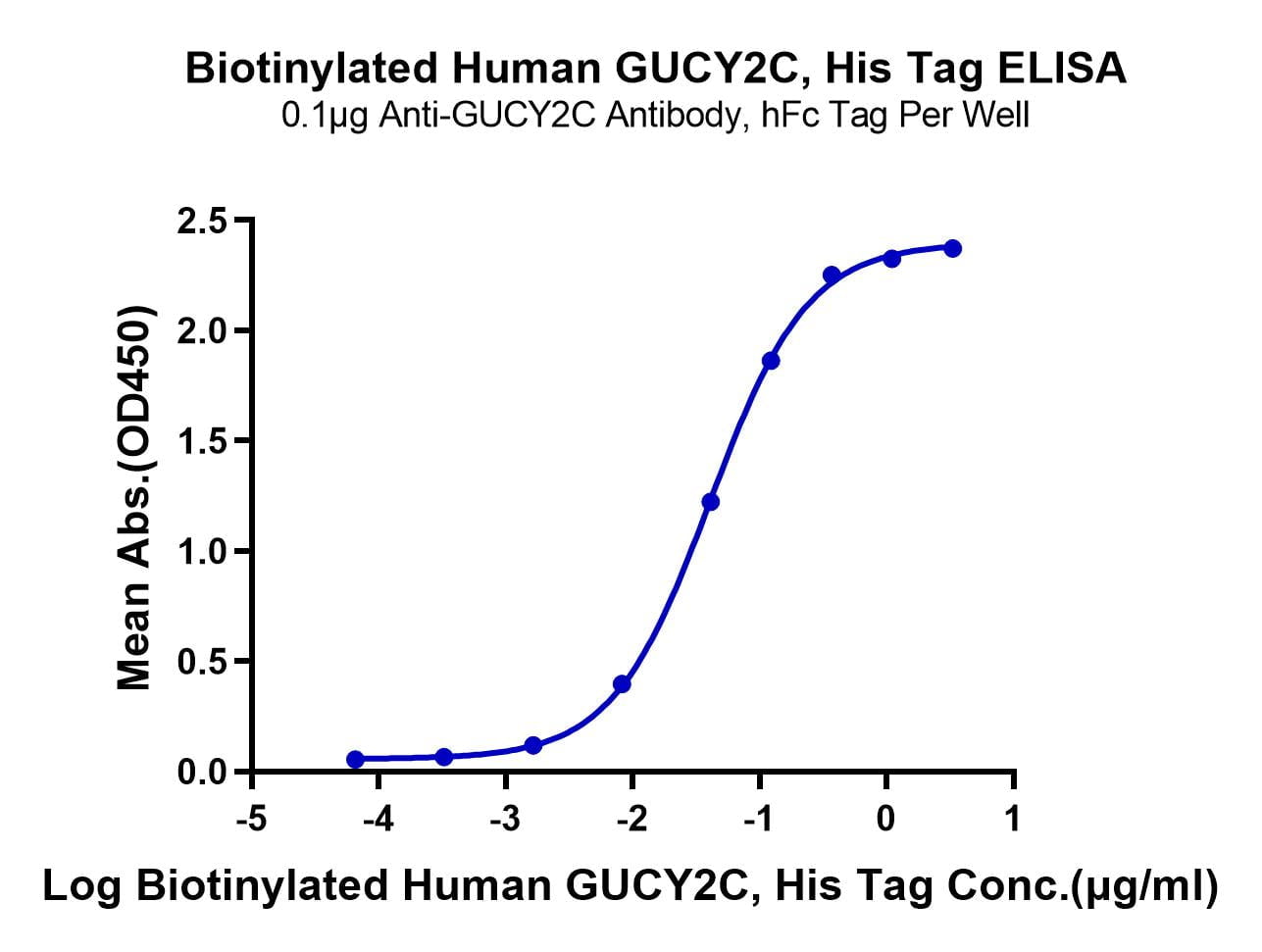
- Functional: Yes (ELISA)
- Amino Acid Range: Ser24-Gln430
Biotinylated Human GUCY2C/Guanylyl cyclase C Protein 3381
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Guanylyl cyclase C (GUCY2C) has canonical centrality in defense of key intestinal homeostatic mechanisms, encompassing fluid and electrolyte balance, epithelial dynamics, antitumorigenesis, and intestinal barrier function. GUCY2C may represent a new target for anti-obesity pharmacotherapy. |
| Protein names Guanylyl cyclase C (GC-C) (EC 4.6.1.2) (Heat-stable enterotoxin receptor) (STA receptor) (hSTAR) (Intestinal guanylate cyclase) |
| Protein family 317 |
| Function Guanylyl cyclase that catalyzes synthesis of cyclic GMP (cGMP) from GTP (PubMed:11950846, PubMed:1718270, PubMed:22436048, PubMed:22521417, PubMed:23269669). Receptor for the E.coli heat-stable enterotoxin; E.coli enterotoxin markedly stimulates the accumulation of cGMP in mammalian cells expressing GUCY2C (PubMed:1680854, PubMed:1718270). Also activated by the endogenous peptides guanylin and uroguanylin (PubMed:8381596). |
| Catalytic activity CATALYTIC ACTIVITY: Reaction=GTP = 3',5'-cyclic GMP + diphosphate; Xref=Rhea:RHEA:13665, ChEBI:CHEBI:33019, ChEBI:CHEBI:37565, ChEBI:CHEBI:57746; EC=4.6.1.2; Evidence=; PhysiologicalDirection=left-to-right; Xref=Rhea:RHEA:13666; Evidence=; |
| Pathway 211 |
| Subellular location Cell membrane ; Single-pass type I membrane protein. Endoplasmic reticulum membrane ; Single-pass type I membrane protein. Note=The 145 kDa plasma membrane form of GUCY2C contains sialic acid and galactose residues, while a differencially glycosylated 130 Kda form is a high mannose form that is resident in the endoplasmic reticulum and may serve as the precursor for the cell surface form. |
| Structure Homotrimer (PubMed:11123935). Interacts via its C-terminal region with NHERF4 (PubMed:11950846). Interacts with the lectin chaperone VIP36 (PubMed:23269669). |
| Post-translational modification Glycosylation at Asn-75 and/or Asn-79 is required for interaction with VIP36 while glycosylation at Asn-345 and Asn-402 modulates ligand-mediated GUCY2C activation. |
| Domain The protein kinase domain is predicted to be catalytically inactive. |
| Target Relevance information above includes information from UniProt accession: P25092 |
| The UniProt Consortium |
Data
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||