| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | P21981 |
| express system | E.coli |
| product tag | N-His |
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | Transglutaminase 2 (TGM2) is a member of the transglutaminase family, and it is reported to be associated with chemoresistance in various types of cancer. TGM2 was demonstrated to affect the chemosensitivity of osteosarcoma cells via regulation of the activation of mitogen‑activated protein kinase and AKT serine/threonine kinase pathways. |
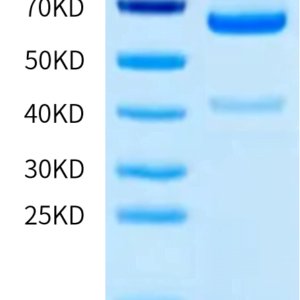
| molecular weight | The protein has a predicted MW of 78.2 kDa same as Tris-Bis PAGE result. |
| available size | 100 µg, 500 µg |
| endotoxin | Less than 1EU per ug by the LAL method. |
Mouse TGM2 Protein 3374
$300.00 – $1,000.00
Summary
- Expression: E.coli
- Pure: Yes (HPLC)
- Amino Acid Range: Ala2-Ala686
Mouse TGM2 Protein 3374
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Transglutaminase 2 (TGM2) is a member of the transglutaminase family, and it is reported to be associated with chemoresistance in various types of cancer. TGM2 was demonstrated to affect the chemosensitivity of osteosarcoma cells via regulation of the activation of mitogen-activated protein kinase and AKT serine/threonine kinase pathways. |
| Protein names Protein-glutamine gamma-glutamyltransferase 2 (EC 2.3.2.13) (Isopeptidase TGM2) (EC 3.4.-.-) (Protein-glutamine deamidase TGM2) (EC 3.5.1.44) (Protein-glutamine dopaminyltransferase TGM2) (EC 2.3.1.-) (Protein-glutamine histaminyltransferase TGM2) (EC 2.3.1.-) (Protein-glutamine noradrenalinyltransferase TGM2) (EC 2.3.1.-) (Protein-glutam |
| Gene names Tgm2,Tgm2 |
| Protein family Transglutaminase superfamily, Transglutaminase family |
| Mass 77061Da |
| Function Calcium-dependent acyltransferase that catalyzes the formation of covalent bonds between peptide-bound glutamine and various primary amines, such as gamma-amino group of peptide-bound lysine, or mono- and polyamines, thereby producing cross-linked or aminated proteins, respectively (By similarity). Involved in many biological processes, such as bone development, angiogenesis, wound healing, cellular differentiation, chromatin modification and apoptosis (By similarity). Acts as a protein-glutamine gamma-glutamyltransferase by mediating the cross-linking of proteins, such as ACO2, HSPB6, FN1, HMGB1, RAP1GDS1, SLC25A4/ANT1, SPP1 and WDR54 (PubMed:11113189, PubMed:11274171, PubMed:20489165). Under physiological conditions, the protein cross-linking activity is inhibited by GTP; inhibition is relieved by Ca(2+) in response to various stresses (By similarity). When secreted, catalyzes cross-linking of proteins of the extracellular matrix, such as FN1 and SPP1 resulting in the formation of scaffolds (By similarity). Plays a key role during apoptosis, both by (1) promoting the cross-linking of cytoskeletal proteins resulting in condensation of the cytoplasm, and by (2) mediating cross-linking proteins of the extracellular matrix, resulting in the irreversible formation of scaffolds that stabilize the integrity of the dying cells before their clearance by phagocytosis, thereby preventing the leakage of harmful intracellular components (PubMed:12810961, PubMed:15905580). In addition to protein cross-linking, can use different monoamine substrates to catalyze a vast array of protein post-translational modifications: mediates aminylation of serotonin, dopamine, noradrenaline or histamine into glutamine residues of target proteins to generate protein serotonylation, dopaminylation, noradrenalinylation or histaminylation, respectively (PubMed:32116663). Mediates protein serotonylation of small GTPases during activation and aggregation of platelets, leading to constitutive activation of these GTPases (By similarity). Plays a key role in chromatin organization by mediating serotonylation and dopaminylation of histone H3 (By similarity). Catalyzes serotonylation of 'Gln-5' of histone H3 (H3Q5ser) during serotonergic neuron differentiation, thereby facilitating transcription (By similarity). Acts as a mediator of neurotransmission-independent role of nuclear dopamine in ventral tegmental area (VTA) neurons: catalyzes dopaminylation of 'Gln-5' of histone H3 (H3Q5dop), thereby regulating relapse-related transcriptional plasticity in the reward system (By similarity). Regulates vein remodeling by mediating serotonylation and subsequent inactivation of ATP2A2/SERCA2 (PubMed:32116663). Also acts as a protein deamidase by mediating the side chain deamidation of specific glutamine residues of proteins to glutamate (By similarity). Catalyzes specific deamidation of protein gliadin, a component of wheat gluten in the diet (By similarity). May also act as an isopeptidase cleaving the previously formed cross-links (By similarity). Also able to participate in signaling pathways independently of its acyltransferase activity: acts as a signal transducer in alpha-1 adrenergic receptor-mediated stimulation of phospholipase C-delta (PLCD) activity and is required for coupling alpha-1 adrenergic agonists to the stimulation of phosphoinositide lipid metabolism (PubMed:11274171). |
| Catalytic activity CATALYTIC ACTIVITY: Reaction=L-glutaminyl-[protein] + L-lysyl-[protein] = [protein]-L-lysyl-N(6)-5-L-glutamyl-[protein] + NH4(+); Xref=Rhea:RHEA:54816, Rhea:RHEA-COMP:9752, Rhea:RHEA-COMP:10207, Rhea:RHEA-COMP:14005, ChEBI:CHEBI:28938, ChEBI:CHEBI:29969, ChEBI:CHEBI:30011, ChEBI:CHEBI:138370; EC=2.3.2.13; Evidence=; PhysiologicalDirection=left-to-right; Xref=Rhea:RHEA:54817; Evidence=; CATALYTIC ACTIVITY: Reaction=L-glutaminyl-[protein] + serotonin = 5-serotonyl-L-glutamyl-[protein] + NH4(+); Xref=Rhea:RHEA:66552, Rhea:RHEA-COMP:10207, Rhea:RHEA-COMP:17052, ChEBI:CHEBI:28938, ChEBI:CHEBI:30011, ChEBI:CHEBI:167174, ChEBI:CHEBI:350546; Evidence=; PhysiologicalDirection=left-to-right; Xref=Rhea:RHEA:66553; Evidence=; CATALYTIC ACTIVITY: Reaction=dopamine + L-glutaminyl-[protein] = 5-dopaminyl-L-glutamyl-[protein] + NH4(+); Xref=Rhea:RHEA:66556, Rhea:RHEA-COMP:10207, Rhea:RHEA-COMP:17053, ChEBI:CHEBI:28938, ChEBI:CHEBI:30011, ChEBI:CHEBI:59905, ChEBI:CHEBI:167175; Evidence=; PhysiologicalDirection=left-to-right; Xref=Rhea:RHEA:66557; Evidence=; CATALYTIC ACTIVITY: Reaction=histamine + L-glutaminyl-[protein] = 5-histaminyl-L-glutamyl-[protein] + NH4(+); Xref=Rhea:RHEA:66564, Rhea:RHEA-COMP:10207, Rhea:RHEA-COMP:17056, ChEBI:CHEBI:28938, ChEBI:CHEBI:30011, ChEBI:CHEBI:58432, ChEBI:CHEBI:167179; Evidence=; PhysiologicalDirection=left-to-right; Xref=Rhea:RHEA:66565; Evidence=; CATALYTIC ACTIVITY: Reaction=(R)-noradrenaline + L-glutaminyl-[protein] = 5-(R)-noradrenalinyl-L-glutamyl-[protein] + NH4(+); Xref=Rhea:RHEA:66560, Rhea:RHEA-COMP:10207, Rhea:RHEA-COMP:17054, ChEBI:CHEBI:28938, ChEBI:CHEBI:30011, ChEBI:CHEBI:72587, ChEBI:CHEBI:167178; Evidence=; PhysiologicalDirection=left-to-right; Xref=Rhea:RHEA:66561; Evidence=; CATALYTIC ACTIVITY: Reaction=H2O + L-glutaminyl-[protein] = L-glutamyl-[protein] + NH4(+); Xref=Rhea:RHEA:16441, Rhea:RHEA-COMP:10207, Rhea:RHEA-COMP:10208, ChEBI:CHEBI:15377, ChEBI:CHEBI:28938, ChEBI:CHEBI:29973, ChEBI:CHEBI:30011; EC=3.5.1.44; Evidence=; PhysiologicalDirection=left-to-right; Xref=Rhea:RHEA:16442; Evidence=; |
| Subellular location Cytoplasm, cytosol. Nucleus. Chromosome. Secreted, extracellular space, extracellular matrix. Cell membrane. Mitochondrion. Note=Mainly localizes to the cytosol. Present at much lower level in the nucleus and chromatin. Also secreted via a non-classical secretion pathway to the extracellular matrix. |
| Structure Monomer. Interacts with phospholipase C; promoting alpha-1 adrenergic receptor signaling (By similarity). Interacts with PLCD1 (By similarity). |
| Post-translational modification Disulfide bond formation inactivates the calcium-dependent acyltransferase activity. Cys-370 can form disulfide bonds with both Cys-230 and Cys-371: formation of a disulfide bond between Cys-230 and Cys-370 facilitates formation of the disulfide between Cys-370 and Cys-371, which promotes inactivation of the acyltransferase activity. May also form interchain disulfids between Cys-230 and Cys-370. Ca(2+) protects against disulfide bond formation and inactivation.; Auto-transglutaminated: Forms covalent cross-links mediated by transglutaminase between Gln-632 and the epsilon-amino group of a lysine residue of itself or HMGB1, forming homopolymers and heteropolymers, respectively.; S-nitrosylated, leading to inactivation of the acyltransferase activity. |
| Target Relevance information above includes information from UniProt accession: P21981 |
| The UniProt Consortium |
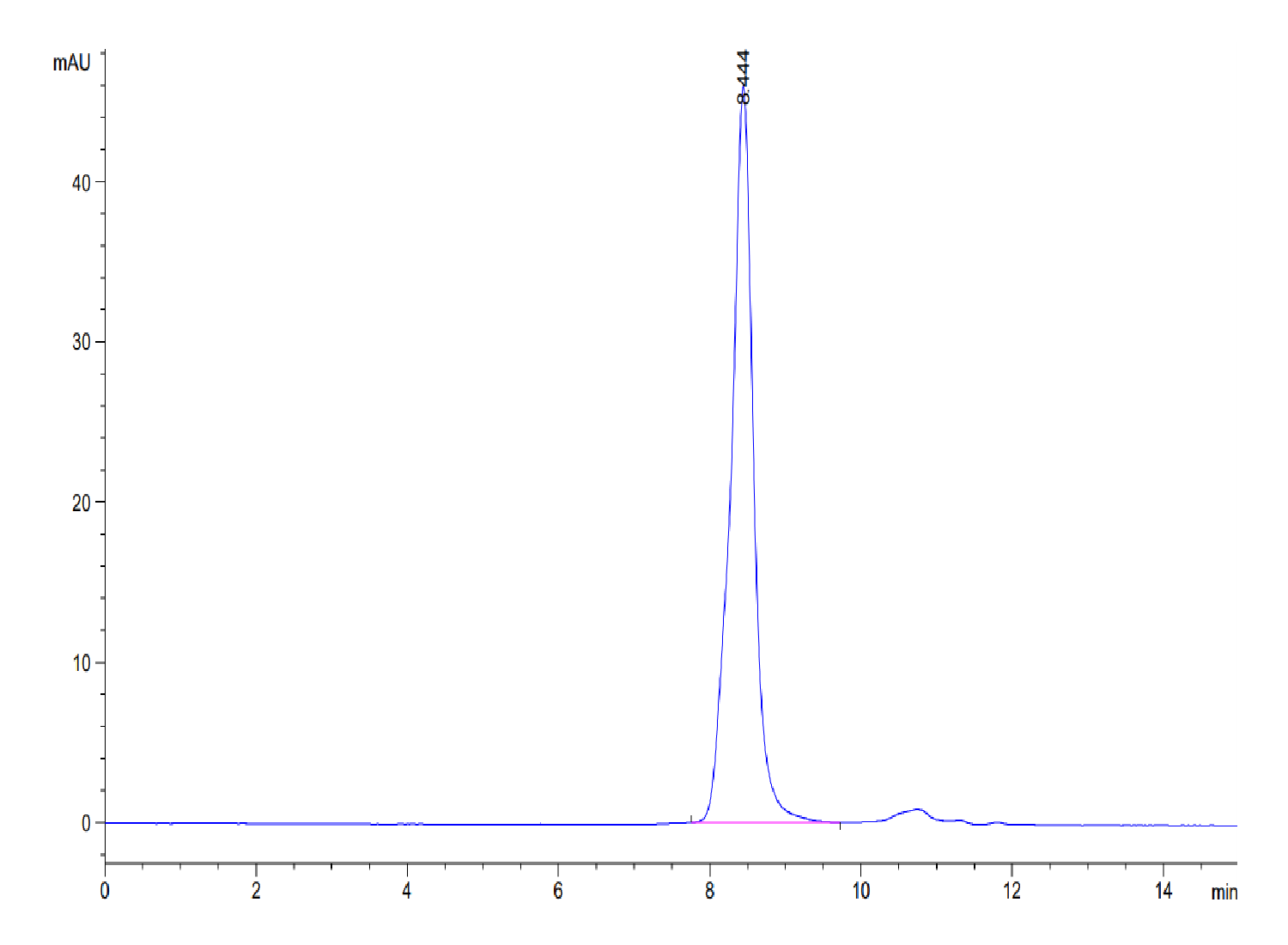 |
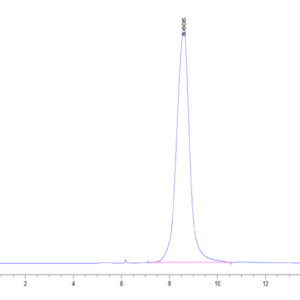
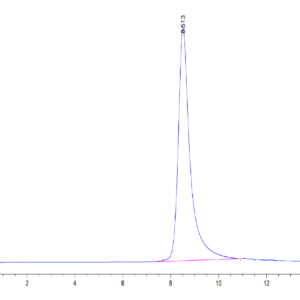
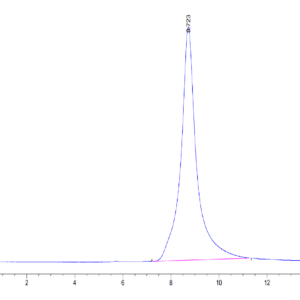
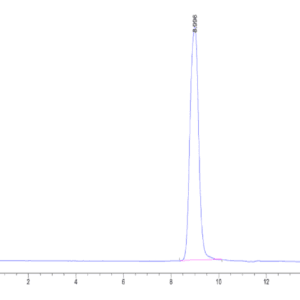
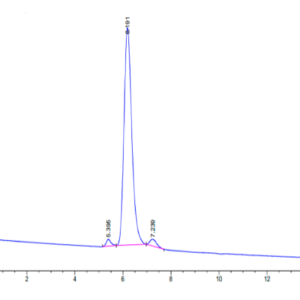
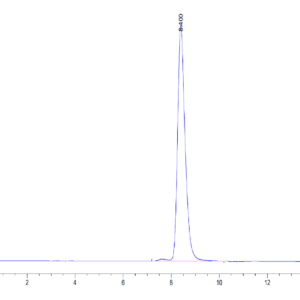
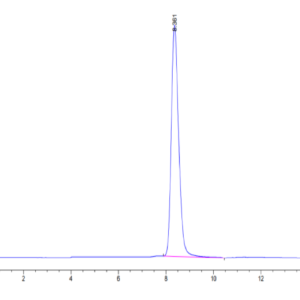
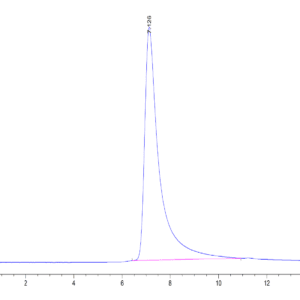
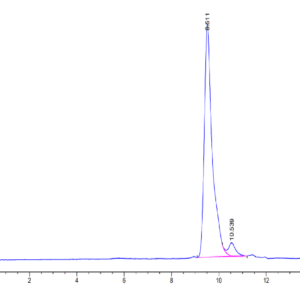
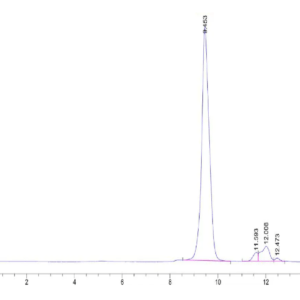
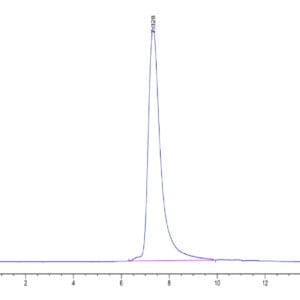
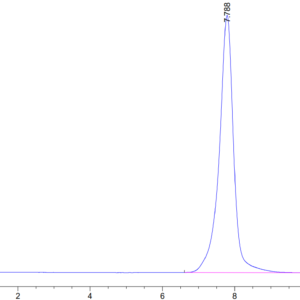
| The purity of Mouse TGM2 is greater than 95% as determined by SEC-HPLC. |
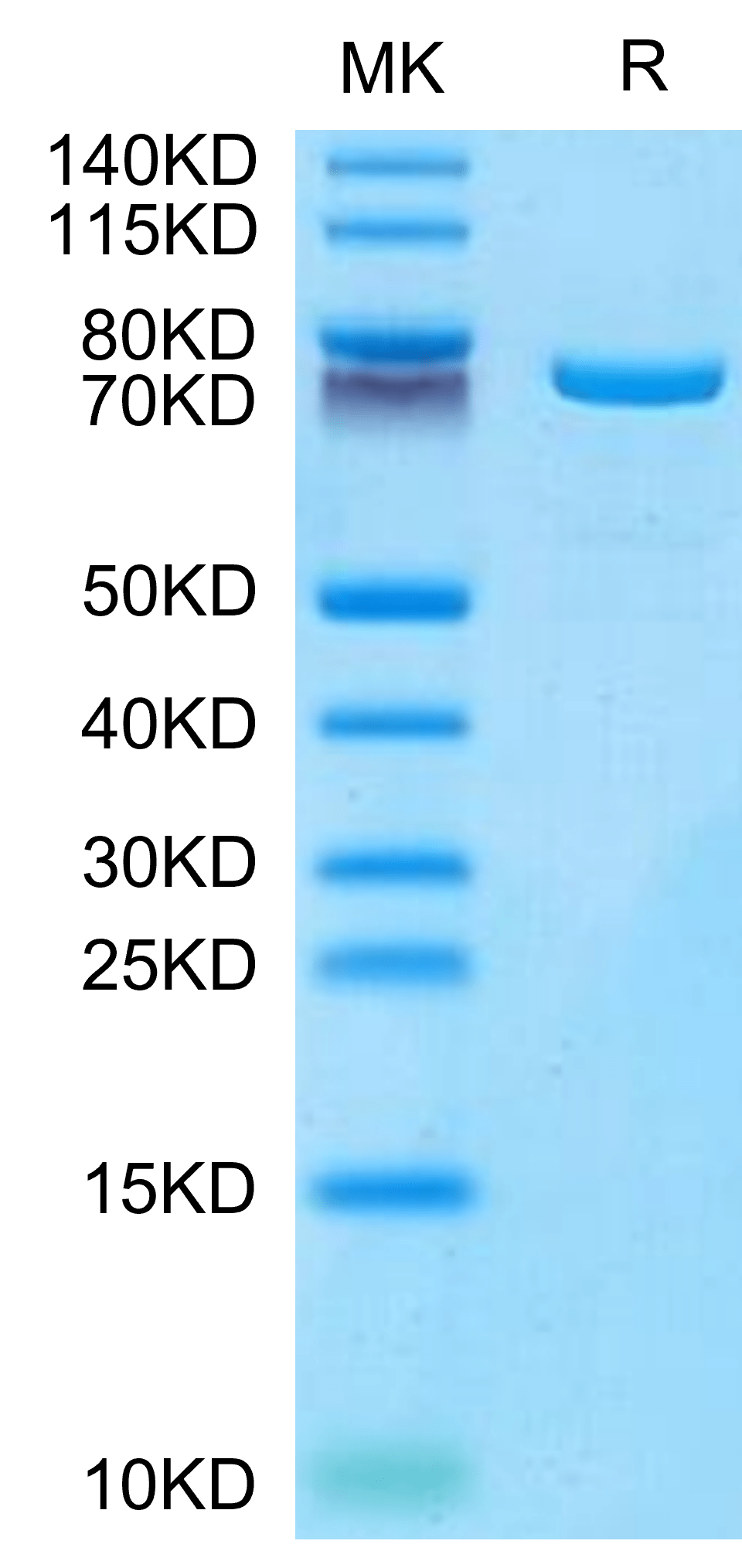 |
| Mouse TGM2 on Tris-Bis PAGE under reduced condition. The purity is greater than 95%. |
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
| relevant to this product |
|---|
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||
Only logged in customers who have purchased this product may leave a review.
Reviews
There are no reviews yet.