| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| accession | NP_075213.2 |
| express system | HEK293 |
| product tag | C-His |
| purity | > 95% as determined by Tris-Bis PAGE;> 95% as determined by HPLC |
| background | Prothrombin, or coagulation factor II, is a multidomain zymogen precursor of thrombin that undergoes an allosteric equilibrium between two alternative conformations, open and closed, that react differently with the physiological activator prothrombinase. It has 10 sites of gamma-carboxylation, which are required for its bioactivity, and is N-glycosylated at three of four putative sites. |
| molecular weight | The protein has a predicted MW of 69.12 kDa. Due to glycosylation, the protein migrates to 70-80 kDa based on Tris-Bis PAGE result. |
| available size | 100 Āµg, 500 Āµg |
| endotoxin | Less than 1EU per Ī¼g by the LAL method. |
Rat Coagulation Factor IIĀ Protein 5035
$315.00 – $1,050.00
Summary
- Expression: HEK293
- Pure: Yes (HPLC)
- Amino Acid Range: Gln25-Arg617
Rat Coagulation Factor IIĀ Protein 5035
| protein |
|---|
| Size and concentration 100, 500µg and lyophilized |
| Form Lyophilized |
| Storage Instructions Valid for 12 months from date of receipt when stored at -80°C. Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
| Storage buffer Shipped at ambient temperature. |
| Purity > 95% as determined by Tris-Bis PAGE |
| target relevance |
|---|
| Prothrombin, or coagulation factor II, is a multidomain zymogen precursor of thrombin that undergoes an allosteric equilibrium between two alternative conformations, open and closed, that react differently with the physiological activator prothrombinase. It has 10 sites of gamma-carboxylation, which are required for its bioactivity, and is N-glycosylated at three of four putative sites. |
| Protein names Prothrombin (EC 3.4.21.5) (Coagulation factor II) [Cleaved into: Activation peptide fragment 1; Activation peptide fragment 2; Thrombin light chain; Thrombin heavy chain] |
| Protein family Peptidase S1 family |
| Mass 70037Da |
| Function Thrombin, which cleaves bonds after Arg and Lys, converts fibrinogen to fibrin and activates factors V, VII, VIII, XIII, and, in complex with thrombomodulin, protein C. Functions in blood homeostasis, inflammation and wound healing. Thrombin triggers the production of pro-inflammatory cytokines, such as MCP-1/CCL2 and IL8/CXCL8, in endothelial cells (PubMed:30568593, PubMed:9780208). {ECO:0000269|PubMed:2856554, ECO:0000269|PubMed:30568593, ECO:0000269|PubMed:9780208}. |
| Catalytic activity CATALYTIC ACTIVITY: Reaction=Selective cleavage of Arg-|-Gly bonds in fibrinogen to form fibrin and release fibrinopeptides A and B.; EC=3.4.21.5; |
| Subellular location Secreted, extracellular space. |
| Tissues Expressed by the liver and secreted in plasma. |
| Structure Heterodimer (named alpha-thrombin) of a light and a heavy chain; disulfide-linked. Forms a heterodimer with SERPINA5. In plasma, interacts (via N-terminus) with alpha-1-microglobulin with molar ratio 1:2 and 1:1; this interaction does not prevent the activation of prothrombin to thrombin. Interacts (thrombin) with iripin-8, a serine protease inhibitor from Ixodes ricinus saliva (PubMed:34502392). Interacts (thrombin) with iripin-3, a serine protease inhibitor from Ixodes ricinus saliva (PubMed:33732248). {ECO:0000269|PubMed:11493008, ECO:0000269|PubMed:16763681, ECO:0000269|PubMed:17685615, ECO:0000269|PubMed:18291642, ECO:0000269|PubMed:18362344, ECO:0000269|PubMed:2369893, ECO:0000269|PubMed:2374926, ECO:0000269|PubMed:33732248, ECO:0000269|PubMed:34502392, ECO:0000269|PubMed:9183005}. |
| Post-translational modification The gamma-carboxyglutamyl residues, which bind calcium ions, result from the carboxylation of glutamyl residues by a microsomal enzyme, the vitamin K-dependent carboxylase. The modified residues are necessary for the calcium-dependent interaction with a negatively charged phospholipid surface, which is essential for the conversion of prothrombin to thrombin. {ECO:0000269|PubMed:3759958, ECO:0000269|PubMed:6305407}.; N-glycosylated. N-glycan heterogeneity at Asn-121: Hex3HexNAc3 (minor), Hex4HexNAc3 (minor) and Hex5HexNAc4 (major). At Asn-143: Hex4HexNAc3 (minor) and Hex5HexNAc4 (major). {ECO:0000269|PubMed:14760718, ECO:0000269|PubMed:16335952, ECO:0000269|PubMed:19139490, ECO:0000269|PubMed:19159218, ECO:0000269|PubMed:19838169, ECO:0000269|PubMed:22171320, ECO:0000269|PubMed:873923}.; In the penultimate step of the coagulation cascade, prothrombin is converted to thrombin by the prothrombinase complex composed of factor Xa (F10), cofactor Va (F5), and phospholipids. This activation requires factor Xa-catalyzed sequential cleavage at 2 sites, Arg-314 and Arg-363, along 2 possible pathways. In the first pathway, the first cleavage occurs at Arg-314, leading to the formation of the inactive intermediate prethrombin-2. This pathway preferentially occurs on platelets and in the absence of cofactor Va. In the second pathway, the first cleavage occurs at Arg-363, which separates protease domain into 2 chains that remain connected through a disulfide bond and generates the active intermediate meizothrombin. The presence of cofactor Va directs activation along the meizothrombin pathway and greatly accelerates the rate of cleavage at Arg-363, but has a smaller effect on the cleavage of meizothrombin at Arg-314. Meizothrombin accumulates as an intermediate when prothrombinase is assembled on the membrane of red blood cells. {ECO:0000305|PubMed:34265300}. |
| Target Relevance information above includes information from UniProt accession: P00734 |
| The UniProt Consortium |
Data
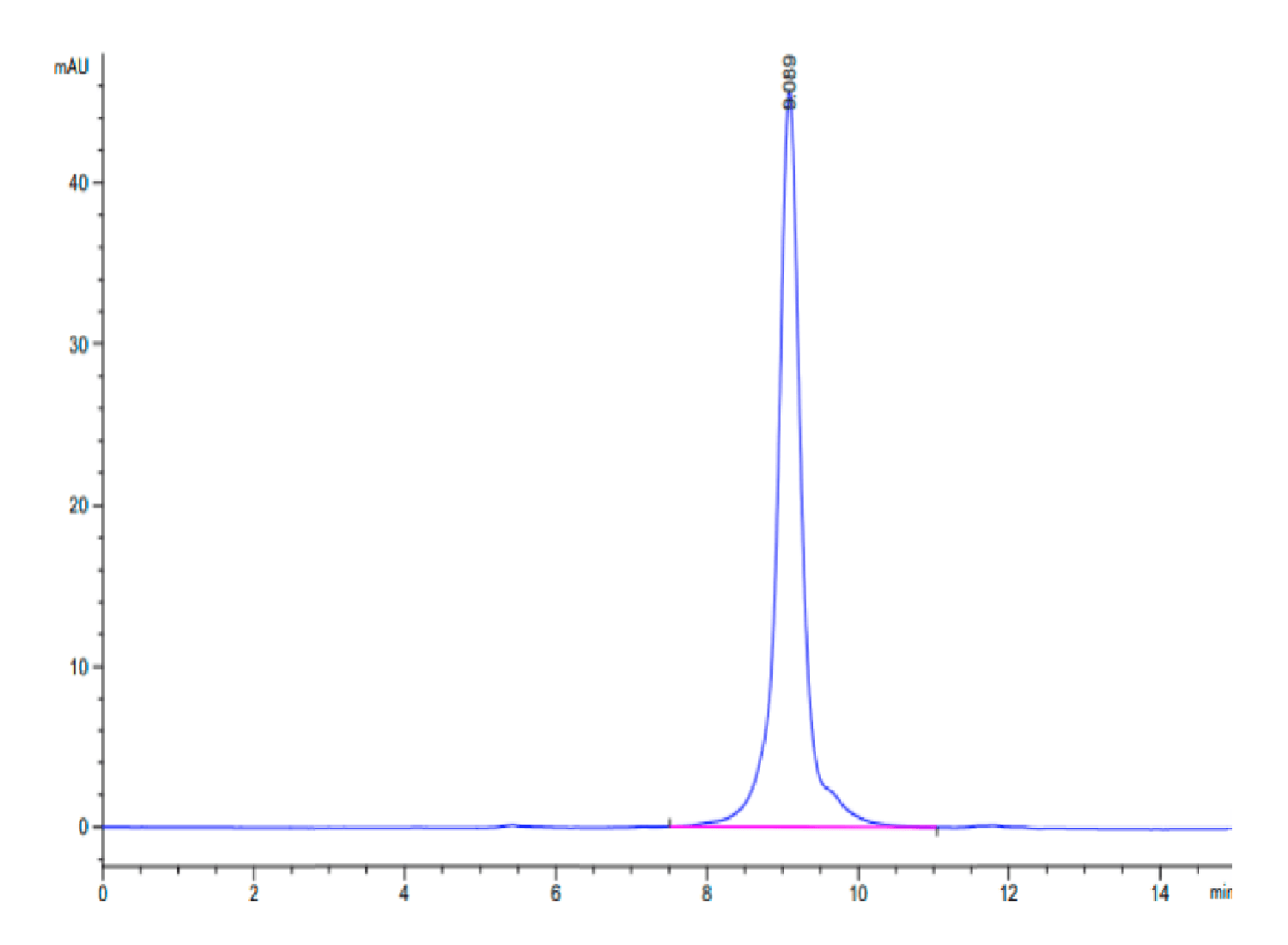 |
| The purity of Rat Coagulation Factor II is greater than 95% as determined by SEC-HPLC. |
 |
| Rat Coagulation Factor II on Tris-Bis PAGE under reduced condition. The purity is greater than 95%. |
Publications
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||














