| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| target | Human Epstein–Barr virus IgG |
| species reactivity | Human Epstein–Barr virus |
| applications | Lateral flow (dipstick) |
| assay type | Indirect & qualitative |
| available sizes | 20 test kits |
Human Epstein–Barr virus IgG Lateral flow dipstick kit 4572
$487.00
Summary
- Mikrogen diagnostik lateral flow device (dipstick) for research use (RUO)
- Human Epstein–Barr virus IgG Lateral flow dipstick kit 4572
- Suitable for IgG detection
- Ready-to-use
- 20 tests
Human Epstein–Barr virus IgG Lateral flow dipstick kit 4572
| kit | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay type Sandwich assay, lateral flow (dipstick) | ||||||||||||||||||||||||||||||||
| Research area Infectious Disease | ||||||||||||||||||||||||||||||||
| Sample type Serum, plasma, whole blood | ||||||||||||||||||||||||||||||||
Components
| ||||||||||||||||||||||||||||||||
| Storage Store at 2-8°C. | ||||||||||||||||||||||||||||||||
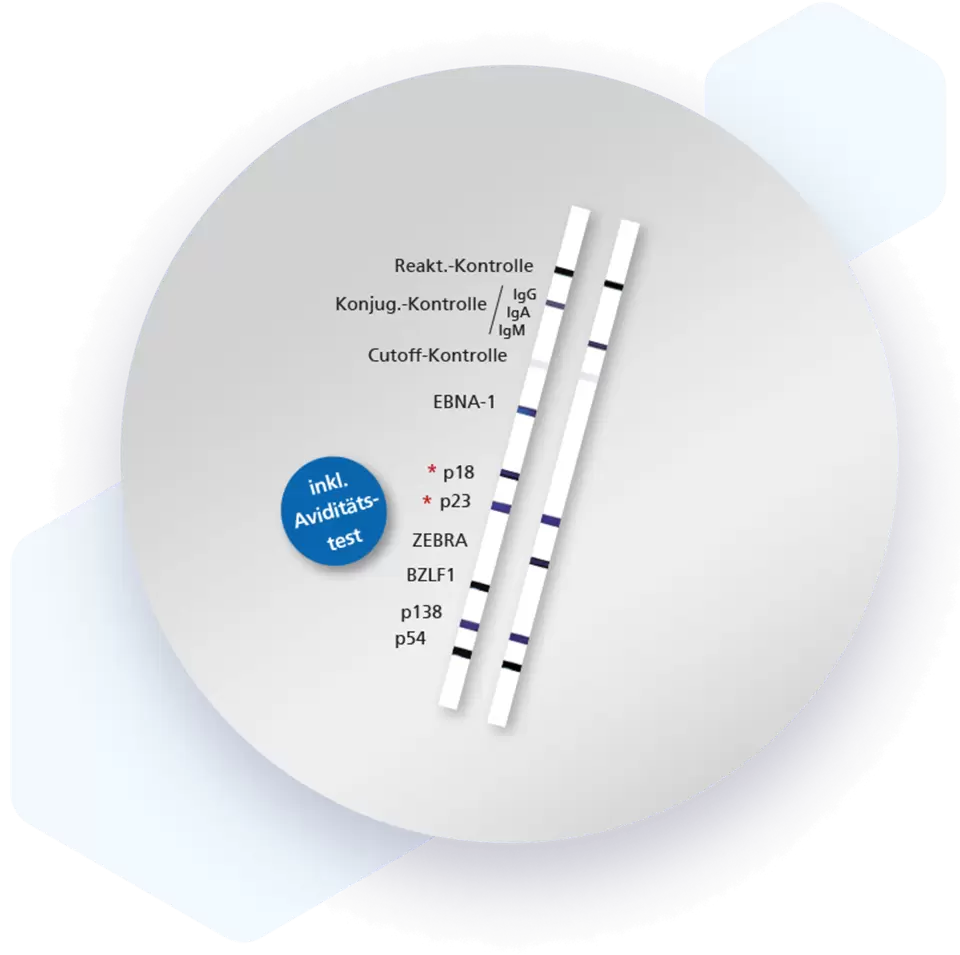
| Additional information Mikrogen recomLine EBV tests are serological, qualitative in vitro line immunoassays based on recombinantly produced, highly specific and characteristic EBV antigens. They are designed as screening tests. The line assay technique allows the detection and identification of IgG and IgM antibodies against the different antigen classes in one approach and at one glance. Advantages
|
| target relevance |
|---|
| Organism Epstein-Barr Virus |
| Protein names Epstein-Barr Virus |
| Structure and strains The Epstein Barr virus (EBV), formally called Human gammaherpesvirus 4, is one of the nine known human herpesvirus types in the herpes family, and is one of the most common viruses in humans. EBV is a double-stranded DNA virus. Epstein-Barr virus (EBV) is the first identified oncogenic virus, which establishes permanent infection in humans. EBV causes infectious mononucleosis and is also tightly linked to many malignant diseases. Various vaccine formulations underwent testing in different animals or in humans. However, none of them was able to prevent EBV infection and no vaccine has been approved to date. The virus causes infectious mononucleosis ("mono" or "glandular fever"). It is also associated with various non-malignant, premalignant, and malignant Epstein Barr virus-associated lymphoproliferative diseases such as Burkitt lymphoma, hemophagocytic lymphohistiocytosis, and Hodgkin's lymphoma; non-lymphoid malignancies such as gastric cancer and nasopharyngeal carcinoma; and conditions associated with human immunodeficiency virus such as hairy leukoplakia and central nervous system lymphomas. The virus is also associated with the childhood disorders of Alice in Wonderland syndrome and acute cerebellar ataxia and, by some evidence, higher risks of developing certain autoimmune diseases, especially dermatomyositis, systemic lupus erythematosus, rheumatoid arthritis, and Sjogren's syndrome. About 200,000 cancer cases globally per year are thought to be attributable to EBV. In 2022, a large study (population of 10 million over 20 years) suggested EBV as the leading cause of multiple sclerosis, with a recent EBV infection causing a 32-fold increase in the risk of developing multiple sclerosis. |
| Detection and diagnosis IgG antibodies directed against early antigen (EA) and/or IgM antibodies against virus capsid antigens (VCA) in combination with negative EBNA1 IgG activity serve as evidence of acute primary infections. High IgG antibody activities against EBNA1 exclude acute primary infections. IgG antibodies directed against VCA can usually be detected life-long and serve as confirmation of contact with the pathogen. In reactivations, high IgG antibody activities directed against VCA and EA can frequently be found. |
Data
Publications
Warning: Cannot modify header information - headers already sent by (output started at /www/benchmarkantibodiescom_769/public/wp-includes/script-loader.php:3015) in /www/benchmarkantibodiescom_769/public/wp-content/plugins/shortcode-manager/shortcode-manager.php(453) : eval()'d code on line 3
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| 4572 protocol |
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||
Only logged in customers who have purchased this product may leave a review.









Reviews
There are no reviews yet.