| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| target | Human Helicobacter IgA |
| species reactivity | Human Helicobacter |
| applications | Lateral flow (dipstick) |
| assay type | Indirect & qualitative |
| available sizes | 20 test kits |
Human Helicobacter IgA Lateral flow dipstick kit 4775
$487.00
Summary
- Mikrogen diagnostik lateral flow device (dipstick) for research use (RUO)
- Human Helicobacter IgA Lateral flow dipstick kit 4775
- Suitable for IgA detection
- Ready-to-use
- 20 tests
Human Helicobacter IgA Lateral flow dipstick kit 4775
| kit | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay type Sandwich assay, lateral flow (dipstick) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Research area Infectious Disease | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sample type Serum, plasma, whole blood | ||||||||||||||||||||||||||||||||||||||||||||||||
Components
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Storage Store at 2-8°C. | ||||||||||||||||||||||||||||||||||||||||||||||||
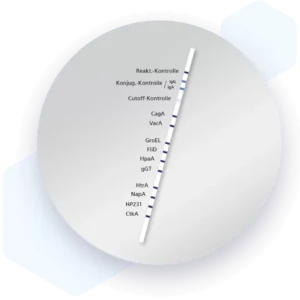
| Additional information Mikrogen recomLine Helicobacter 2.0 tests are serological, qualitative in vitro line immunoassays for the detection of antibodies against ten selected Helicobacter pylori antigens. The detection of antibodies against the two most important virulence factors CagA and VacA are of high diagnostic importance. These two virulence factors significantly increase the risk for premalignant changes, gastric carcinoma, MALT lymphoma, and ulcers. Humoral immune responses against the GroEL, HtrA, NapA, HP231, and CtkA biomarkers are associated with more severe disease progression. Advantages
DataPublications
Protocols
Documents
Only logged in customers who have purchased this product may leave a review. | ||||||||||||||||||||||||||||||||||||||||||||||||








Reviews
There are no reviews yet.