Fluorescence is a captivating phenomenon that has transformed the way we label antibodies and image cells. To comprehend this mesmerizing effect, one must grasp the fundamental principles governing it: excitation, excited state, decay, and emission, and a crucial factor called the Stokes shift. These principles are intricately linked to the relationship between energy and color, unraveling the mysteries of fluorescence.
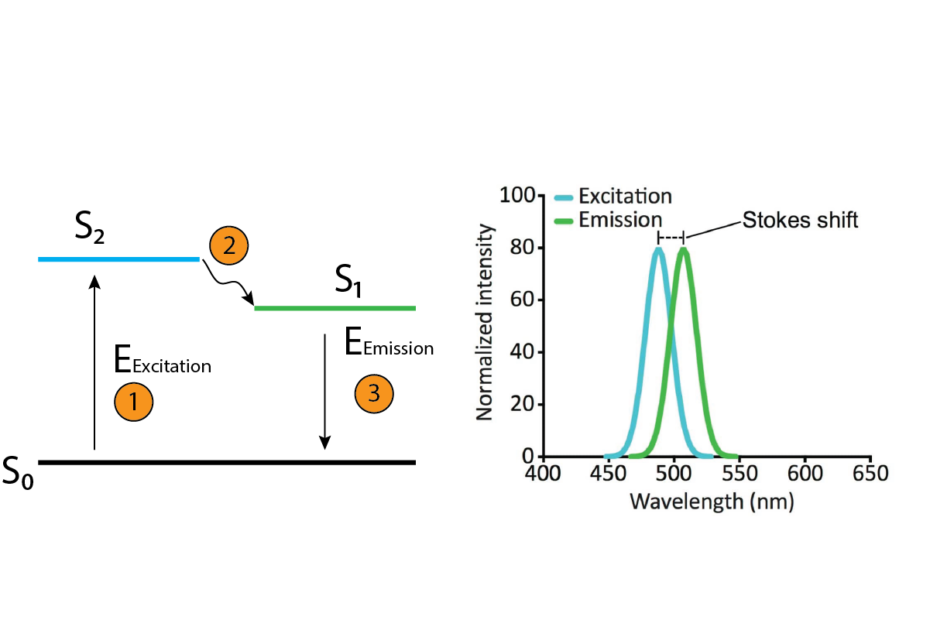
1) Excitation: The journey of fluorescence commences with excitation. This process involves exposing a substance, typically a fluorescent molecule or compound, to a specific wavelength of light. This light carries energy that is absorbed by the electrons within the molecule, causing them to jump to a higher energy level, or “excited state.” The absorbed energy is crucial; it determines the eventual color of the emitted light and is defined by Planck’s equation, E=hf, where E is energy, h is Planck’s constant, and f is the frequency of the excitation light.
2) Stokes Shift: Enter the Stokes shift, a pivotal concept in fluorescence. As the excited electrons begin their journey back to the ground state, they release the surplus energy they previously absorbed. This energy is typically emitted as light. The crucial distinction here is that the emitted light carries less energy than the absorbed light, resulting in a longer wavelength and, consequently, a lower energy photon. This deviation from the excitation wavelength is known as the Stokes shift. It plays a pivotal role in determining the color of the emitted light. The larger the Stokes shift, the more distinct the color difference between the excitation and emission.
3) Emission: Finally, the electrons return to their ground state, releasing energy in the form of photons. The color of this emitted light is the hallmark of a particular fluorophore, and it’s contingent on the size of the Stokes shift. A larger energy difference between the excited and ground states corresponds to a shorter wavelength and higher energy photons, yielding colors like blue or violet. Conversely, a smaller energy difference results in longer wavelengths, generating colors like red or orange.
Now, let’s address the question of why different fluorophores exhibit varying colors. The answer lies in the molecular structure of the fluorophores themselves. The arrangement of atoms within a molecule, its electronic properties, and its energy levels determine the precise energy difference between the excited and ground states. These intrinsic characteristics dictate the color a fluorophore emits during fluorescence. Consequently, different fluorophores possess unique molecular signatures, leading to a stunning diversity of colors in the world of fluorescence.
In essence, the principles of fluorescence, together with the Stokes shift, offer a comprehensive framework for understanding the intricate relationship between energy and color in this mesmerizing phenomenon. This understanding not only fuels scientific exploration but also enriches our appreciation of the vibrant spectrum of fluorescence that surrounds us, from natural bioluminescence to the precise fluorescence imaging techniques utilized in scientific research and medical diagnostics.