This is the first in our series of blog posts discussing Nanobodies, the petite powerhouses of the biotechnology world, are intriguing not only for their origins but also for their remarkable sequence and domain structure. These miniature antibodies, found in animals like llamas and camels, have captivated the scientific community with their unique attributes. In this blog post, we’ll take a closer look at the fascinating sequence and domain structure of nanobodies.
The Building Blocks
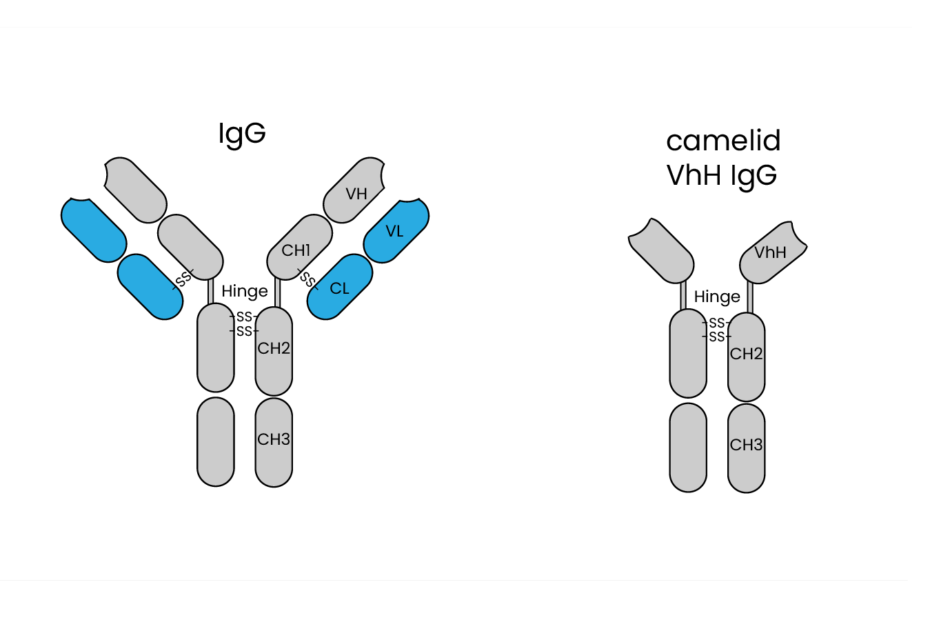
Nanobodies are constructed from the genetic sequences that code for their protein components. Unlike traditional antibodies composed of two heavy chains and two light chains, nanobodies are derived from only the variable heavy-chain domain of conventional antibodies. This single-domain structure is a hallmark of nanobodies and is primarily responsible for their distinct properties.
Sequencing Nanobodies
Sequencing nanobodies is a crucial step in their characterization. By examining their genetic makeup, scientists can identify the specific amino acid sequences responsible for binding to target antigens. This knowledge enables researchers to manipulate nanobodies for various applications, including therapeutics, diagnostics, and structural biology.
Simplified isolation, library creation and manipulation
The unique single-domain structure, which offers several advantages over traditional antibodies such as IgG. Unlike IgG antibodies, which consist of two heavy chains and two light chains, nanobodies are single polypeptide chains that possess a complete, functional binding domain. This characteristic simplifies the antibody development process significantly. Traditional antibody development requires the challenging task of matching sequences between heavy and light chains to ensure proper antibody function. In contrast, nanobodies eliminate the need for such sequence matching, streamlining the process and reducing the potential for errors in construction. This attribute enables researchers to create a library of single-domain sequences with ease, providing a diverse and versatile resource for antibody development.
Furthermore, the single-domain nature of nanobodies simplifies the process of selecting and optimizing binders for specific targets. With a library of these single sequences at their disposal, researchers can use a technique called “panning” to identify nanobodies that bind strongly to a desired target. This efficient screening process allows for the rapid identification of potent candidates for further development. Additionally, the genetic modification and optimization of nanobodies can be carried out more straightforwardly compared to traditional antibodies, making it easier to enhance their affinity, specificity, and other properties. The combination of a complete sequence, simplified library generation, and efficient modification processes makes nanobodies an indispensable tool for antibody development and a promising asset in various biomedical applications.
Comprehensive Diversity
The diversity of nanobodies is a testament to the adaptability of their DNA sequences. Llamas and camels, the primary sources of nanobodies, have evolved to produce an impressive array of unique heavy-chain antibodies. Through the examination of nanobody sequences, scientists have discovered a vast library of potential binders to an extensive range of antigens.
Single-Domain Structure
The single-domain structure of nanobodies sets them apart from traditional antibodies. These nanobodies consist of a single, compact protein domain, often referred to as the VHH domain. This unique structure offers several advantages. It allows nanobodies to access hidden epitopes on target molecules, which can be challenging for larger antibodies. The single domain also contributes to their small size, typically around 15 kDa, making them highly soluble and stable biomolecules.
Tailoring Nanobodies
The beauty of nanobodies lies in their ability to be engineered. Researchers can manipulate the sequences of these single domains to create nanobodies with tailored binding capabilities. This opens the door to the development of highly specific and efficient tools for various applications, such as targeted therapies and diagnostic assays.
A Glimpse into the Future
Nanobodies’ unique sequence and domain structure continue to be a source of fascination and exploration. As we gain a deeper understanding of their DNA sequences and genetic makeup, we are on the cusp of unlocking their full potential in the fields of medicine, biotechnology, and structural biology.
In conclusion, nanobodies are much more than just tiny antibodies; they are intricate marvels of molecular biology. Their distinctive sequence and domain structure, derived from nature’s own adaptations, offer an exciting avenue for scientific discovery and innovation. As we continue to decode their DNA and harness their remarkable properties, the future holds endless possibilities for the application of these miniature wonders.