Introduction
Within the microscopic world of cells lies a complex framework known as the cytoskeleton, like the human skeleton it provides structural support but also orchestrates various cellular activities. At the heart of this cytoskeletal network are actin filaments, slender threads of protein that play a fundamental role in shaping the cell and enabling essential functions like cell movement, phagocytosis, and cell division. In this blog post, we will take a deep dive into the fascinating world of actin filaments, exploring their structure, organization, regulation, and vital contributions to cell biology.
The Structure and Organization of Actin Filaments
Actin filaments, also known as microfilaments, are approximately 7 nanometers in diameter and can stretch several micrometers in length. These delicate threads are the primary cytoskeletal proteins in most cells, where they are organized into higher-order structures. Actin filaments are not haphazardly distributed but form intricate networks and bundles, lending mechanical support and determining the shape of the cell.
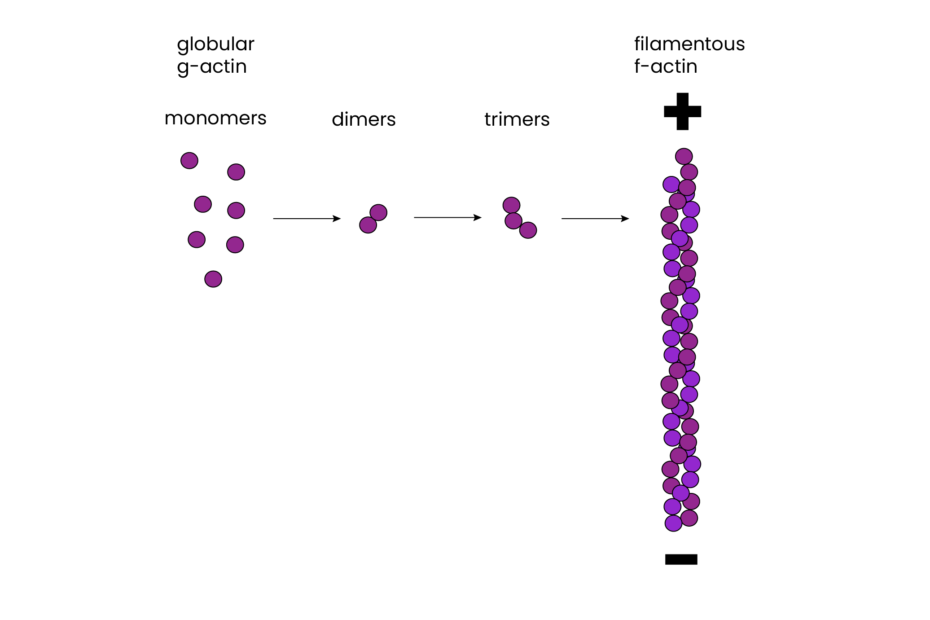
Actin filaments, composed of individual globular actin monomers (G-actin), exhibit a distinct polarity. They have plus and minus ends, with all monomers oriented in the same direction. This polarity is vital for both filament assembly and guiding myosin movement relative to actin, a critical aspect of cellular functions.
Assembly and Disassembly of Actin Filaments
The assembly of actin filaments is a dynamic process regulated by various actin-binding proteins. Actin monomers polymerize to form filaments, with ATP binding enhancing polymerization. In vitro studies have revealed that the formation of small aggregates, called nucleation, is the first step in actin polymerization. Once nucleation occurs, filaments can grow by the reversible addition of monomers to both ends, though the plus end elongates significantly faster than the minus end.
One intriguing feature of actin filaments is their ability to undergo treadmilling. Treadmilling is a dynamic behavior where monomers are added to the plus end while dissociating from the minus end. This process is dependent on ATP and plays a role in cell movement and shape changes.
Regulation by Actin-Binding Proteins
Actin filaments’ assembly and disassembly are tightly regulated by a cadre of actin-binding proteins. Notably, cofilin promotes filament disassembly and can sever actin filaments, generating more ends for further disassembly. Profilin, another actin-binding protein, stimulates the incorporation of actin monomers into filaments by facilitating the exchange of bound ADP for ATP.
These actin-binding proteins, along with others like Arp2/3 proteins, work in concert to promote the rapid turnover of actin filaments, allowing cells to efficiently adapt to changes in their environment. The activities of these proteins are controlled by various cell signaling mechanisms, ensuring precise regulation of actin polymerization in response to external cues.
Association of Actin Filaments with the Plasma Membrane
Actin filaments are strategically concentrated beneath the cell’s plasma membrane, forming a three-dimensional network known as the cell cortex. This network not only provides mechanical support but also influences cell shape and enables various surface-related activities.
In red blood cells, for instance, the structural basis of the cortical cytoskeleton is provided by the actin-binding protein spectrin. Spectrin forms tetramers composed of two α and two β chains, which crosslink with actin filaments and attach to the plasma membrane via proteins like ankyrin. This linkage ensures the integrity and stability of the cell’s biconcave shape.
In other cell types, such as fibroblasts, actin filaments attach to the plasma membrane at focal adhesions, where they form stress fibers. These stress fibers exert tension on the cell’s substratum, aiding in cell attachment and movement.
Cell Surface Protrusions
Actin filaments also play a pivotal role in the formation of various cell surface protrusions, including microvilli, pseudopodia, and lamellipodia. Microvilli, found on cells involved in absorption, increase surface area for nutrient uptake. These structures consist of tightly packed actin filaments crosslinked by proteins like villin.
Pseudopodia, on the other hand, are extensions of moderate width formed during phagocytosis or amoeboid movement. Lamellipodia, broad sheet-like extensions, are characteristic of fibroblast cells’ leading edges during migration.
Conclusion
In the complex world of cellular biology, actin filaments stand as the versatile architects of cell structure and function. Their precisely regulated assembly, organization into higher-order structures, and dynamic interactions with the plasma membrane make them indispensable for processes like cell shape determination, movement, and phagocytosis. Understanding the intricate dance of actin filaments not only sheds light on fundamental cellular mechanisms but also opens doors to potential therapeutic interventions in various diseases linked to cytoskeletal dysfunction.