Introduction
The intricate world of cellular biology never ceases to amaze us with its complex processes that keep our bodies running smoothly. One such fascinating mechanism is ubiquitinylation, a cellular process that plays a pivotal role in cellular recycling. Ubiquitinylation, also known as ubiquitination, is a remarkable system that marks specific proteins for degradation and enables the cell to recycle and renew its components. In this blog post, we will explore the importance of ubiquitinylation in cellular recycling and its impact on maintaining cellular health.
Understanding Ubiquitinylation
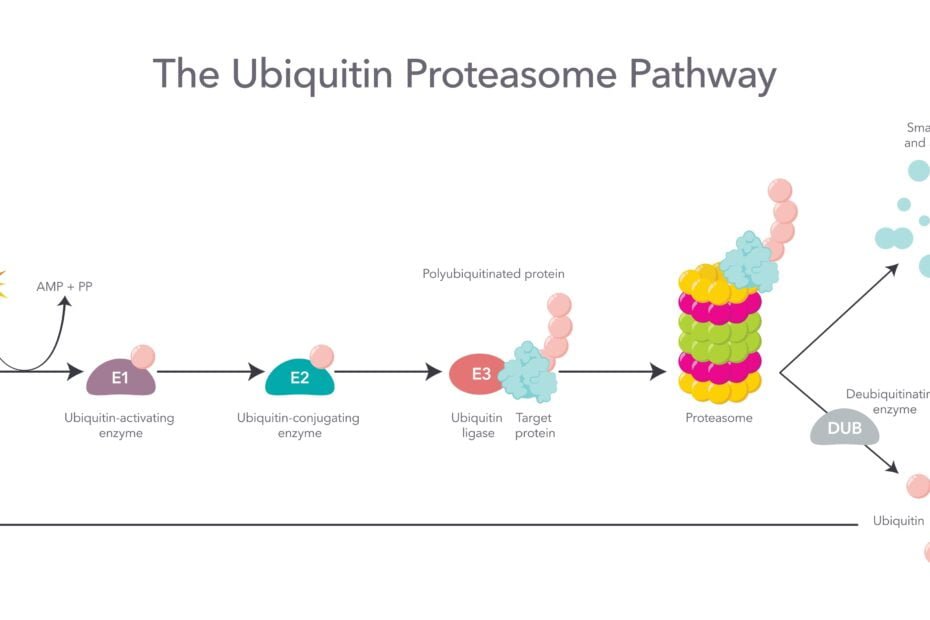
Ubiquitinylation is a post-translational modification process in which a small protein called ubiquitin is attached to target proteins. This attachment occurs via a cascade of enzymatic reactions involving E1, E2, and E3 ubiquitin ligases. The addition of ubiquitin molecules acts as a molecular tag, flagging the protein for degradation within the cell’s proteasome or lysosome, the cellular recycling centers. This tagging is essential for maintaining a dynamic balance in the cell by eliminating damaged or unneeded proteins.
The Proteasome: The Cellular Trash Compactor
The ubiquitinylation system directs proteins to the proteasome, a large protein complex that acts as a cellular garbage disposal unit. Here, the tagged proteins are unfolded, chopped into small peptides, and recycled, ensuring that the cell disposes of misfolded, surplus, or harmful proteins. This process is crucial for maintaining the cell’s health and preventing the accumulation of toxic protein aggregates, which are associated with various diseases, including neurodegenerative conditions like Alzheimer’s and Parkinson’s.
Cellular Renewal and Quality Control
Ubiquitinylation not only aids in protein degradation but also plays a significant role in regulating the cell’s processes. It acts as a quality control mechanism, ensuring that newly synthesized proteins are correctly folded and functional. In cases of protein misfolding or damage, the ubiquitin system rapidly targets the aberrant proteins for degradation, preventing them from interfering with normal cellular functions. This quality control aspect of ubiquitinylation is essential for the overall well-being of the cell.
Autophagy: Cellular Recycling at Its Best
In addition to the proteasome, ubiquitinylation is also involved in the process of autophagy, a cellular recycling system that engulfs and digests damaged organelles and cellular components. Autophagy is vital for maintaining cellular homeostasis and is implicated in various physiological processes, including immune response, aging, and the body’s adaptation to stress conditions. The ubiquitin system tags specific organelles or structures for engulfment by autophagosomes, which then fuse with lysosomes, leading to the degradation and recycling of the contents.
Conclusion
Ubiquitinylation is a remarkable cellular process that serves as the molecular key to cellular recycling. By tagging specific proteins for degradation and regulating the quality of newly synthesized proteins, ubiquitinylation ensures that the cell remains healthy and functional. The interconnected systems of the proteasome and autophagy, driven by ubiquitin, play a vital role in maintaining cellular homeostasis. Understanding this process not only sheds light on the intricacies of cellular biology but also holds promise for developing therapies for various diseases linked to protein misfolding and accumulation. Ubiquitinylation is a testament to the brilliance of nature’s design in orchestrating the recycling and renewal of our cells.