| Weight | 1 lbs |
|---|---|
| Dimensions | 9 × 5 × 2 in |
| target | Campylobacter jejuni reactive IgA |
| species reactivity | Campylobacter jejuni |
| applications | ELISA |
| assay type | Indirect & quantitative |
| available sizes | 96 tests |
Campylobacter jejuni IgA ELISA Kit ESR139A
$398.00
Summary
- Virion/Serion Diagnostic Kit for research use (RUO)
- Campylobacter jejuni IgA ELISA Kit
- Suitable for IgA detection
- Ready-to-use
- 96 tests
Campylobacter jejuni IgA ELISA Kit ESR139A
| kit | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay type Indirect ELISA | ||||||||||||||||||
| Research area Infectious Disease | ||||||||||||||||||
| Sample type Serum, plasma, whole blood | ||||||||||||||||||
Components
| ||||||||||||||||||
| Storage Store at 2-8°C. | ||||||||||||||||||
| Associated products Campylobacter jejuni Antigen (BA139VS) Campylobacter jejuni IgA Control Serum (BC139A) Campylobacter jejuni IgG Control Serum (BC139G) Campylobacter jejuni IgM Control Serum (BC139M) Campylobacter jejuni IgA ELISA Kit (ESR139A) Campylobacter jejuni IgG ELISA Kit (ESR139G) Campylobacter jejuni IgM ELISA Kit (ESR139M) |
| target relevance |
|---|
| Organism Campylobacter jejuni |
| Protein names Campylobacter jejuni |
| Structure and strains Campylobacter jejuni is a species of pathogenic bacteria, one of the most common causes of food poisoning in Europe and in the US. The vast majority of cases occur as isolated events, not as part of recognized outbreaks. Active surveillance through the Foodborne Diseases Active Surveillance Network (FoodNet) indicates that about 20 cases are diagnosed each year for each 100,000 people in the US, while many more cases are undiagnosed or unreported; the CDC estimates a total of 1.5 million infections every year. The European Food Safety Authority reported 246,571 cases in 2018, and estimated approximately nine million cases of human campylobacteriosis per year in the European Union. |
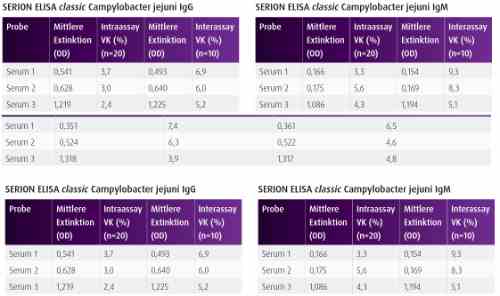
| Detection and diagnosis Campylobacter jejuni infections are usually detected by pathogen isolations from stool and blood samples. In recent years, serological diagnosis of Campylobacter jejuni infections has developed into an important routine diagnostic procedure. To analyze the etiology of severe complications, such as reactive arthritis and GBS, reliable serological ELISA tests are required, since these diseases usually develop up to three weeks after Campylobacter jejuni infection. In such cases, stool culture testing is not suitable since isolation attempts are usually unsuccessful at this stage. |
Data
Publications
| pmid | title | authors | citation |
|---|---|---|---|
| We haven't added any publications to our database yet. | |||
Protocols
| relevant to this product |
|---|
| ESR139A protocol |
Documents
| # | ||
|---|---|---|
| Please enter your product and batch number here to retrieve product datasheet, SDS, and QC information. | ||
Only logged in customers who have purchased this product may leave a review.


Reviews
There are no reviews yet.