Fab fragments, which stand for “Fragment antigen-binding,” are essential components of antibodies that play a pivotal role in various scientific applications. To understand these fragments better, let’s delve into their structure, the process of creating them, and their diverse uses.
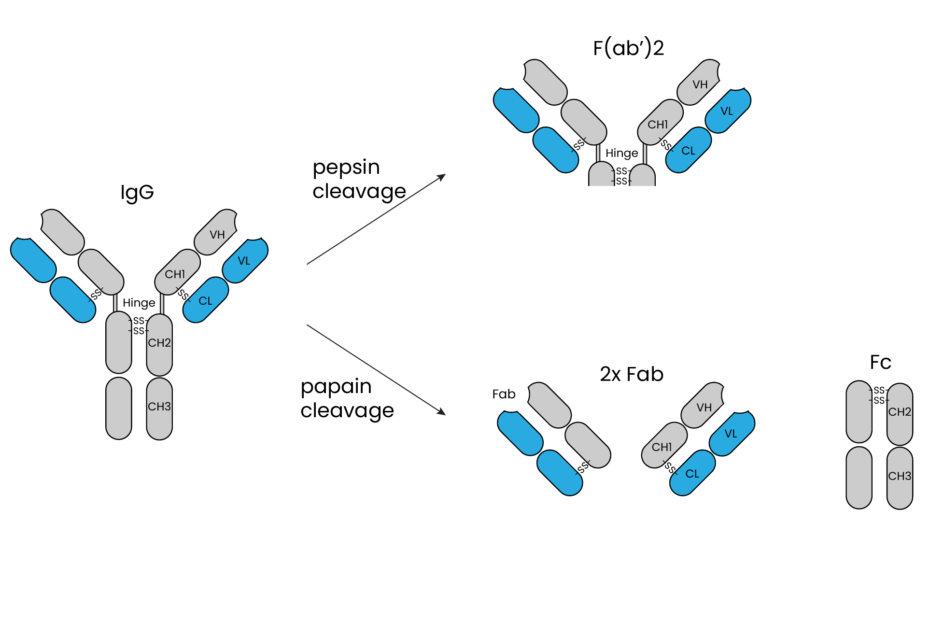
Structure: Fab fragments are derived from the antibody molecule through enzymatic cleavage, specifically at the hinge region. This cleavage divides the antibody into two distinct components: the Fab fragment and the Fc fragment. The Fab fragment retains the variable regions (Fv) of the antibody, which contain the antigen-binding sites responsible for recognizing specific antigens. The Fc fragment, on the other hand, contains the constant regions that are essential for immune effector functions like antibody-dependent cellular cytotoxicity (ADCC) and complement activation.
How to Make Them: The process of generating Fab fragments involves the use of enzymes such as papain or pepsin, which cleave the antibody at specific peptide bonds within the hinge region. Let’s delve into the detailed cleavage sites for both enzymes:
- Papain Cleavage: Papain is a cysteine protease that cleaves antibodies into two Fab fragments and one Fc fragment. It cuts the antibody molecule just below the hinge region, leaving a small piece of the hinge region attached to each Fab fragment. This cleavage occurs at the peptide bonds near the cysteine residues of the hinge region.
- Pepsin Cleavage: Pepsin, an aspartic protease, also cleaves antibodies but generates Fab fragments with a shorter hinge region. Pepsin cleaves the antibody above the hinge region, resulting in Fab fragments that have a truncated hinge. Pepsin cleaves the peptide bonds within the hinge region, but closer to the Fab portion, creating smaller Fab fragments with a more significant loss of the hinge region compared to papain-cleaved Fab fragments.
Uses: Fab fragments find application in various scientific and medical contexts. They are employed in crystallography studies to determine the three-dimensional structures of antigen-antibody complexes, aiding in understanding molecular interactions. In addition to research, Fab fragments are utilized in therapies and diagnostics due to their smaller size, which can enhance tissue penetration and reduce immunogenicity. Techniques like enzyme-linked immunosorbent assays (ELISAs) and flow cytometry make use of Fab fragments for their specific antigen recognition capabilities, allowing precise and sensitive detection. In summary, Fab fragments serve as indispensable tools in biotechnology and medicine, empowering researchers and healthcare professionals to gain deeper insights into immune responses and drive advancements across various scientific disciplines.