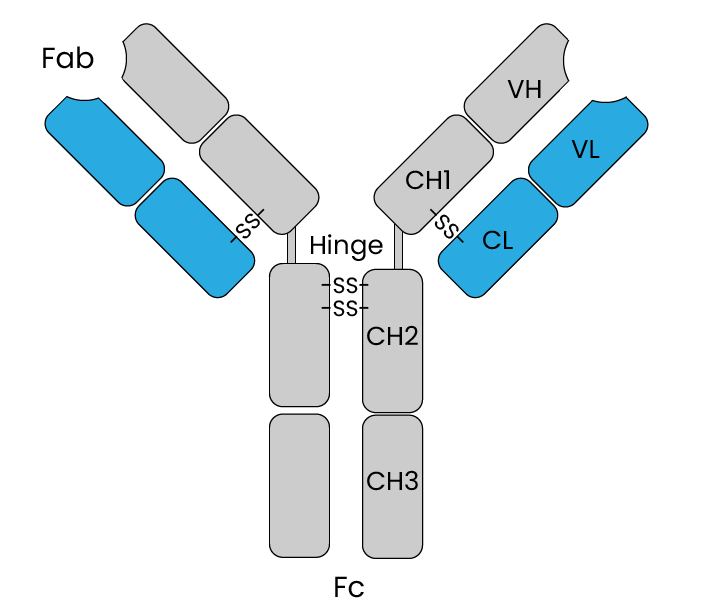
IgG antibodies, a subclass of immunoglobulins, are essential tools in the realm of scientific research, thanks to their remarkable structure and versatility. These antibodies have a well-defined domain organization that allows them to perform various crucial functions. At the core of their structure, IgG antibodies feature two distinct domains: the variable (V) and constant (C) domains, composed of both heavy (H) and light (L) chains. The variable domains, located at the tips of the Y-shaped antibody, are responsible for recognizing and binding to specific antigens, whether they are pathogens, toxins, or other molecules of interest. Within these variable domains lie the complementary determining regions (CDRs), often referred to as the “hypervariable loops.” These CDRs are the key players in antibody specificity, as their unique shapes and amino acid sequences determine the antibody’s ability to precisely target and bind to specific antigens. Meanwhile, the constant domains, found in the antibody’s stem, play a pivotal role in mediating various effector functions, such as activating the immune system or facilitating the destruction of the bound target. Disulfide bonds, formed between specific amino acids in both the heavy and light chains, are instrumental in maintaining the structural integrity of IgG antibodies, ensuring they remain functional.
IgG antibodies are indispensable tools in research due to their ability to bind with high specificity to a wide range of targets. Scientists have harnessed the power of IgG antibodies to probe and manipulate biological systems, whether it’s for the detection of specific proteins in Western blots or immunohistochemistry, the purification of molecules of interest, or the development of therapeutic antibodies for various diseases. The careful understanding of IgG’s domain structure, including the critical role of disulfide bonds in maintaining its shape, empowers researchers to engineer antibodies with precise functionalities. By tailoring the variable domains and CDRs, scientists can create custom-made antibodies designed to interact with specific targets, offering endless possibilities for advancing our knowledge in fields ranging from immunology to molecular biology.