In the world of molecular biology and biomedical research, antibody-horseradish peroxidase (HRP) conjugates have emerged as indispensable tools for various applications, including immunoassays, immunohistochemistry, and Western blotting. To appreciate their significance fully, one must delve into the chemistry of the peroxidase reaction that powers these conjugates. In this blog post, we’ll explore HRP chemistry, shedding light on the catalytic process that makes antibody-HRP conjugates invaluable in the laboratory.
The Enzyme at the Heart of the Reaction
At the core of the antibody-HRP conjugate is horseradish peroxidase, a heme-containing enzyme extracted from the root of the horseradish plant (Armoracia rusticana). HRP is known for its ability to catalyze the oxidation of various substrates, making it a versatile enzyme in biochemical and clinical assays.
Understanding the Peroxidase Reaction
The peroxidase reaction is a multi-step process that involves the enzyme HRP, a substrate (typically hydrogen peroxide, H2O2), and a chromogenic or fluorogenic molecule. Let’s break down the chemistry step by step:
- Formation of Compound I: The peroxidase reaction begins with the binding of H2O2 to the HRP active site. In the presence of an electron donor, typically a phenolic compound, HRP catalyzes the reduction of H2O2 to water and forms an intermediate called Compound I. Compound I is a highly reactive species containing a porphyrin radical cation.
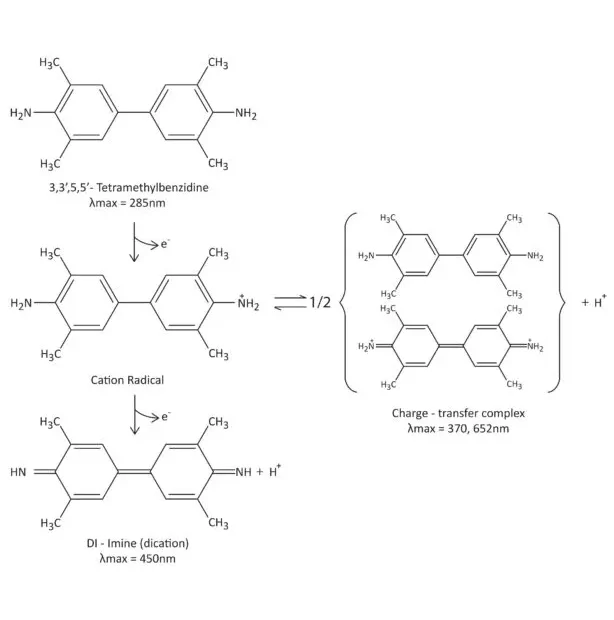
- Compound I Oxidizes Substrate: In the next step, Compound I rapidly oxidizes the chromogenic or fluorogenic substrate. This substrate, often an aromatic compound like 3,3′,5,5′-tetramethylbenzidine (TMB) or 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), serves as the electron donor for the reduction of Compound I. As a result, the substrate becomes oxidized, and Compound I is reduced back to its resting state, known as Compound II.
- Amplification of Signal: The oxidation of the substrate by Compound I produces a detectable signal, such as a color change or fluorescence emission. The intensity of this signal is directly proportional to the amount of HRP present in the conjugate and, therefore, the concentration of the target antigen recognized by the antibody.
Applications of Antibody-HRP Conjugates
Antibody-HRP conjugates are widely used in various laboratory techniques due to their ability to amplify and visualize specific molecular interactions. Here are some common applications:
- Enzyme-Linked Immunosorbent Assay (ELISA): ELISA relies on antibody-HRP conjugates to detect the presence of specific antigens in biological samples. The peroxidase reaction generates a color change that quantifies the concentration of the target molecule.
- Immunohistochemistry (IHC): In IHC, antibody-HRP conjugates are used to visualize the spatial distribution of proteins within tissue samples. The peroxidase reaction produces a brown precipitate at the antigen site, allowing researchers to identify protein localization.
- Western Blotting: Antibody-HRP conjugates are employed to detect specific proteins separated by electrophoresis in Western blotting. The peroxidase reaction generates chemiluminescence or colorimetric signals, enabling the identification of target proteins.
Antibody-HRP conjugates, with their remarkable ability to harness the peroxidase reaction, have become indispensable tools in molecular biology and biomedical research. Understanding the chemistry behind this reaction is crucial for maximizing the utility of these conjugates in various laboratory techniques. As technology continues to advance, antibody-HRP conjugates will likely play an increasingly prominent role in the development of innovative diagnostic assays and research methodologies, paving the way for new discoveries in the field of life sciences.